Modern cataract surgery has come a long way in terms of advancement in techniques, and patient expectations have increased accordingly. Patients want to be as free from glasses as possible for both reading and near. However, there are still limitations with precision and efficiency, and residual astigmatism after cataract surgery results in decreased UCVA and dissatisfied patients. Astigmatic errors of 1.00 D to 2.00 D may reduce BCVA up to 20/50, while errors of 2.00 D to 3.00 D can reduce BCVA up to 20/100.1 Up to 95% of eyes have some degree of measurable, naturally occurring astigmatism.1
Two main approaches for correcting astigmatism during cataract surgery include the use of toric IOLs and the use of corneal relaxing incisions (CRIs). This review discusses the outcomes of relaxing incisions and includes current data based on the new femtosecond laser platforms.
MANUAL PCRIs AND AKs
In cataract surgery. CRIs, which can be performed during cataract surgery to reduce astigmatic error, are divided into two types: peripheral corneal relaxing incisions (PCRIs) and astigmatic keratotomy (AK) incisions. AKs are more centrally located—within an 8-mm optical zone—and, as a result, have more of an effect if all other variables are equal. The incisions result in flattening of the steepest meridian and steepening of the opposite meridian (coupling), and, because the ratio is 1:1 with AKs, they lessen astigmatism without much change in spherical equivalent.2Titiyal et al3 performed 30° manual AKs at the 7-mm optical zone during cataract surgery in 17 patients and showed effective reduction in astigmatism from 1.95 ±0.47 D preoperatively to 0.57 ±0.41 D postoperatively, with 84% eyes achieving less than 1.00 D of residual cylinder.
PCRIs, which were traditionally created manually, are frequently performed in conjunction with cataract surgery and reduce refractive and keratometric corneal cylinder. Wang et al4 reported on a series of 93 eyes treated with combined clear corneal phacoemulsification and PCRIs; at 4 months postoperatively, the percentage of eyes with astigmatism greater than 1.00 D decreased from 51% to 6%. PCRIs can result in a mild hyperopic shift of approximately 0.20 D, which should be considered when selecting the IOL power.5
Manual PCRIs and AKs can be unpredictable given the variations in length, depth, and location, which lead to inaccuracies with nomogram outcomes. They can also result in complications such as infection, wound gape, peripheral scarring, and epithelial ingrowth. Manual AKs originally lost favor to more peripheral relaxing incisions when combined with cataract surgery because they were associated with higher rates of overcorrection and were less reproducible. AKs have a greater effect in older patients and in postkeratoplasty eyes.6
In postkeratoplasty patients. The number one reason for poor vision despite clear penetrating keratoplasty (PKP) is remnant astigmatic error.7 Between 15% and 31% of postkeratoplasty patients may have more than 5.00 D of astigmatism, and as many as 20% have intolerable astigmatism requiring surgical intervention.6,8 When more conventional modalities such as spectacles, rigid contact lenses, and suture removal have failed, AK provides an optimal method to reduce astigmatic errors greater than 4.00 D. Fares et al9 reported 26 post-PKP eyes with astigmatism ranging from 6.00 D to 16.50 D that underwent manual AKs of 80% depth at 7-mm optical zone with coupled compression sutures. The preoperative astigmatism decreased from 9.66 ±2.90 D to 4.37 ±2.53 D at 3 months postoperatively.
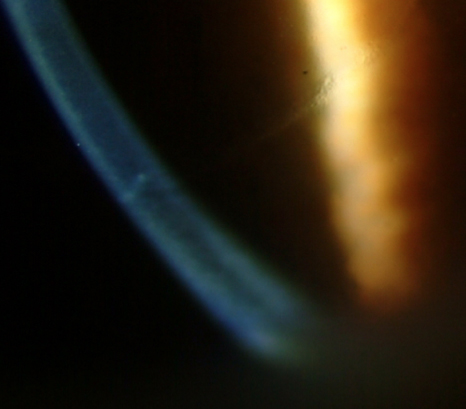
Figure 1 | Slit-lamp photo showing penetrating femtosecond laser AKs.
FEMTOSECOND LASER
Penetrating AKs in concurrent cataract surgery. With the emergence of the femtosecond laser cataract surgery platforms and resultant ability to perform femtosecond laser AKs concurrently with cataract surgery, AKs are again gaining favor. Femtosecond laser AKs are more reproducible and precise in terms of incision depth, length, and centration and, thus, have a lower risk of corneal perforation (Figure 1).6 Intraoperative measurements with OCT imaging during the laser treatment provide real-time feedback to customize the patient’s individual treatment plan.
In a retrospective study, Chan et al10 evaluated the outcomes of 54 eyes that underwent concurrent femtosecond laser-assisted phacoemulsification and AKs using the Victus laser (Bausch + Lomb). The authors found that mean astigmatism decreased from 1.33 ±0.57 D preoperatively to 0.87 ±0.56 D postoperatively. More recently, using the LenSx femtosecond laser (Alcon) to perform cataract surgery and AKs (at the 8-mm optical zone and 90% depth) in 51 eyes, Wang et al11showed a reduction in preoperative astigmatism of 0.65 D. In addition, patient age and AK length and location significantly contributed to the amount of correction.
Penetrating AKs in post-transplant patients. Nubile et al12 reported on the effectiveness of femtosecond laser AKs (Femtec) after PKP in 12 eyes just inside the graft-host junction to a stromal depth of 90%. Patients’ astigmatism was significantly reduced from 7.16 ±3.07 D to 2.23 ±1.55 D and remained stable for several months.
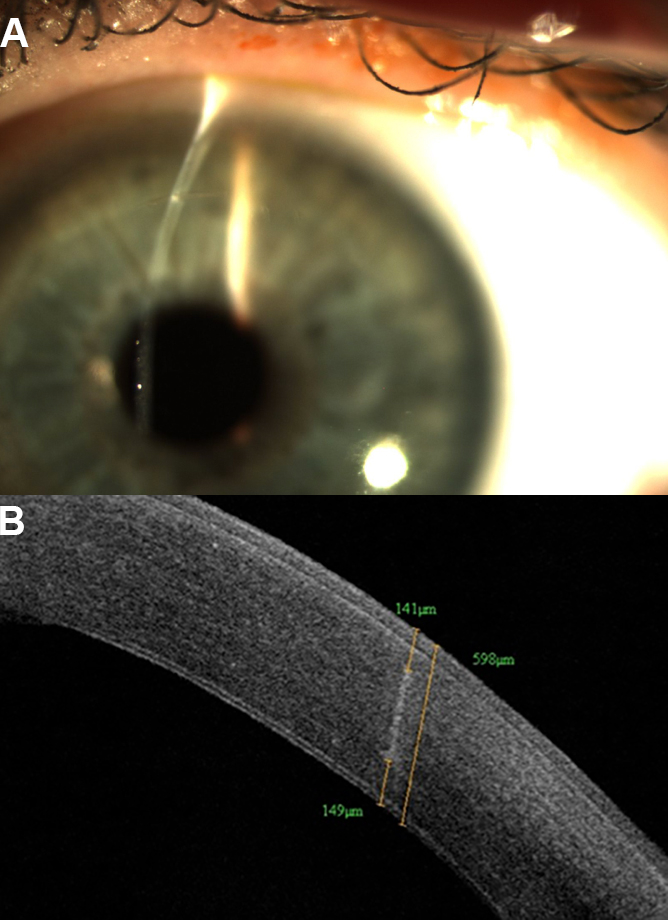
Figure 2 | Slit-lamp photo (A) and anterior segment OCT (B) showing nonpenetrating femtosecond laser intrastromal AKs.
Nonpenetrating AKs in cataract surgery. More recently, nonpenetrating femtosecond laser intrastromal AK (ISAK) has been introduced (Figure 2). Because intrastromal AKs do not penetrate the epithelium, theoretically there is less risk of infection, inflammation, and patient discomfort. In addition, ISAK can potentially result in faster healing and visual recovery compared with penetrating incisions. Multiple studies have shown effective and safe results with ISAK using different femtosecond lasers.
There are less data on the use of the femtosecond laser platforms for concomitant cataract surgery. Day et al13 published a prospective series of 196 eyes that had cataract surgery in addition to ISAK (8-mm optical zone) with the Catalys laser (Abbott Medical Optics). Preoperative astigmatism decreased from 1.21 ±0.42, and the mean difference vector was 0.74 ±0.38 (mean astigmatism correction of 63%). The investigators found that age, astigmatism meridian, corneal hysteresis, and corneal resistance factor were independent predictors of surgically induced astigmatism. There was a 0.09 D regression of treatment at 6 months.14 Our data of 65 eyes who underwent ISAK using the Catalys laser showed that on postoperative anterior segment OCT, the incisions were located more anteriorly than planned with the percentage depth of the midpoint of the incision at 42% instead of the expected 50%, which was statistically significant. Preoperatively, 14% and 38% of patients had corneal astigmatism less than 0.50 D and 0.75 D, respectively. Postoperatively, ISAK increased the percentage of eyes within 0.50 D and 0.75 D of manifest astigmatism to 83% and 91%, respectively.14 Although long-term data are limited, these studies are promising for accurate and safe results.
CONCLUSION
AKs and PCRIs, which can be performed manually or with femtosecond laser technology, are an intrinsic component of refractive cataract surgery as surgeons strive to provide optimal uncorrected vision to patients with preexisting astigmatism. PCRIs and AKs are simple, safe, and quick methods to treat astigmatism. They can be used in virgin eyes, performed in a separate procedure after keratoplasty or refractive surgery, or combined with cataract surgery.
1. Duke-Elder SS, Abrams D. System of Ophthalmology: Ophthalmic Optics and Refraction. St. Louis: Mosby; 1970:274-295.
2. Al-Mohtaseb Z, Raju LV, Wang L, Weikert MP, Koch DD. Incisional keratotomy. In: Holland E, ed. Cornea. 4th edition. Elsevier, 2016. In press.
3. Titiyal JS. Toric intraocular lens implantation versus astigmatic keratotomy to correct astigmatism during phacoemulsification. J Cataract Refract Surg. 2014;40:741-747.
4. Wang L, Misra M, Koch DD. Peripheral corneal relaxing incisions combined with cataract surgery. J Cataract Refract Surg. 2003;29(4):712-722.
5. Wu E. Femtosecond-assisted astigmatic keratotomy. Int Ophthalmol Clin. 2011;51(2)77-85.
6. Price NC, Steele AD. The correction of post-keratoplasty astigmatism. Eye. 1987;1(5):562-566.
7. Javadi MA, Motlagh BF, Jafarinasab MR, et al. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005;24(8):941-946.
8. Fares U. Management of postkeratoplasty astigmatism by paired arcuate incisions with compression sutures. Br J Ophthalmol. 2013;97:438-443.
9. Chan TCY, Cheng GPM, Wang Z, Tham CCY, Woo VCP, Jhanji V. Vector analysis of corneal astigmatism after combined femtosecond-assisted phacoemulsification and arcuate keratotomy. Am J Ophthalmol. 2015;160(2):250-255.
10. Wang L, Zhang S, Zhang Z, et al. Femtosecond laser penetrating corneal relaxing incisions combined with cataract surgery. J Cataract Refract Surg. In press.
11. Nubile M, Carpineto P, Lanzini M, et al. Femtosecond laser arcuate keratotomy for the correction of high astigmatism after keratoplasty. Ophthalmology. 2009;116(6):1083-1092.
12. Day AC. Nonpenetrating femtosecond laser intrastromal astigmatic keratotomy in eyes having cataract surgery. J Cataract Refract Surg. 2016;42:102-109.
13. Day AC, Stevens JD. Predictors of femtosecond laser intrastromal stigmatic keratotomy efficacy for astigmatism management in cataract surgery. J Cataract Refract Surg. 2016;42:251-257.
14. Al-Mohtaseb Z, Koch D, Wang L. Efficacy of femtosecond intrastromal corneal incisions made during cataract surgery. Paper presented at: American Society of Cataract and Refractive Surgery; April 17-21, 2015; San Diego, CA.