After cataract surgery, patients are required to use postoperative drops to help prevent infection and alleviate pain and inflammation. Many patients grow frustrated by the required duration and frequency of these drops, and their surgeons, by the ensuing lack of compliance. Over time, different strategies have been developed and utilized to help reduce the burden of this postoperative drug regimen.
A new intracanalicular dexamethasone-eluting insert, Dextenza (Ocular Therapeutix; Figure), safely and effectively delivers a 4-week tapered release of the corticosteroid to the ocular surface. Dextenza is a noninvasive single-application insert that is placed in the lacrimal canaliculus, which swells and conforms to the canalicular anatomy to ensure insert retention. It is composed of micronized dexamethasone particles suspended within a polyethylene glycol hydrogel matrix. The insert contains 0.4 mg of dexamethasone.
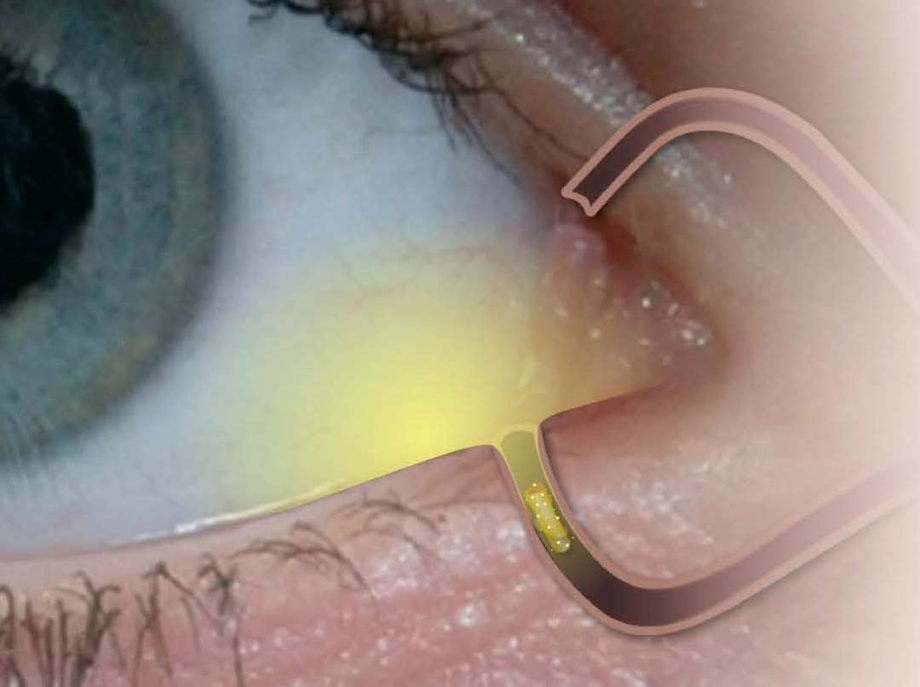
Figure | Intracanalicular depot.
The insert is easy to place and doesn’t have issues with retention. In fact, it only takes about 30 seconds to place the insert intraoperatively, and the insert is conjugated with fluorescein to facilitate visualization throughout the treatment period using a slit lamp with a blue light. Patients report that they cannot feel the insert during placement or postoperatively, and the insert provides a long-term tapered dose of steroid for up to 30 days. After therapy is complete, the hydrogel insert resorbs and exits the nasolacrimal system without the need for removal by the physician.
My involvement with Dextenza occurred during its phase 3a clinical trial. In this study, we found that there was a statistically significant improvement in both pain and inflammation with the insert compared with placebo. As part of the study, we were only allowed to enroll one eye, and many patients complained about having to use drops for their second eye after cataract surgery. They wished we could have placed an insert in their other eye as well.
From a regulatory perspective, in late July 2016, Ocular Therapeutix received a complete response letter from the FDA for its new drug application for Dextenza. One outstanding item pertaining to the manufacturing process and controls was identified. No safety or efficacy issues were raised by the FDA. The company remains optimistic that by working collaboratively with the FDA, Dextenza will be approved soon.
As surgeons incorporate the extended-release dexamethasone insert into their therapeutic regimens, patients could benefit in two important ways. First, the insert ensures that the appropriate dose of corticosteroid is achieved in the anterior chamber throughout the perioperative healing period, with minimal patient effort and input. Second, if used with other pharmacologic agents, patient outcomes may be improved due to precise drug delivery with less overall exposure to corticosteroid as compared with a monthly topical corticosteroid drop regimen.



