Please see Important Safety Information about Oxervate
Inside the Supplement
Neurotrophic Keratitis: A Challenging Disease to Manage
The content from this supplement was derived from a roundtable discussion at the 2019 American Academy of Ophthalmology’s annual meeting in San Francisco. The panelists practice primarily in clinics that receive a high number of referrals for a number of cornea-related issues, including neurotrophic keratitis. All panelists are paid participants.
Marjan Farid, MD: Our discussion today focuses on neurotrophic keratitis, a progressive, degenerative corneal disease caused by impairment of trigeminal innervation. Neurotrophic keratitis leads to corneal epithelial breakdown, compromised healing, and corneal ulceration, melting, and perforation. The hallmark of neurotrophic keratitis is reduced or absent corneal sensation.3,4
Neurotrophic keratitis has been classified as a rare disease and is estimated to affect fewer than 65,000 people in the United States. The ability of the only FDA-approved medication indicated for the treatment of neurotrophic keratitis, cenegermin bkbj 0.002% (20 mcg/mL) ophthalmic solution (Oxervate, Dompé), to target corneal healing reinforces the benefit of early detection and treatment of this potentially debilitating disease.2,3,5
Neurotrophic keratitis Prevalence
Dr. Farid: Neurotrophic keratitis has orphan disease designation; however, based on the experiences of these panelists, I believe some cornea practices are encountering it more often than estimated. At the University of California, Irvine, tertiary care referral practice, I see neurotrophic keratitis every day in the clinic. Is this a rarely seen disease in your practice, Dr. Rapuano?
Christopher J. Rapuano, MD: A rare disease in my practice is one that we see once a year or every other year. A high percentage of my patients have herpes simplex and, to a lesser degree, herpes zoster, and most of them have neurotrophic keratitis. I see it every day.
Kenneth A. Beckman, MD: In my experience, the prevalence does seem much higher than reported in literature. In my practice, which is heavily oriented toward dry eye, a significant number of patients have neurotrophic keratitis.
Francis S. Mah, MD: Part of our challenge when identifying neurotrophic keratitis is that so many factors are associated with it, including LASIK and other eye surgeries, chronic use of topical ocular medications and some systemic medications, as well as systemic diseases, such as diabetes and multiple sclerosis.4
Dr. Farid: As diagnosis of neurotrophic keratitis increases, we will start to see patients with mild disease in addition to those with moderate to severe disease.
Marguerite B. McDonald, MD: I find mild neurotrophic keratitis in many patients who are using more than one glaucoma drop and in people who have been wearing contact lenses for decades.4 Many of the baby boomers I see are now developing neurotrophic keratitis.
Dr. Beckman: Patients with neurotrophic keratitis are also less likely to see an eye doctor, because they’re typically asymptomatic.4
Neurotrophic Keratitis Etiologies
Dr. Farid: A whole host of etiologies—chief among them, herpes simplex and herpes zoster—ultimately lead to impairment of trigeminal innervation of the cornea, resulting in decreased corneal sensation and neurotrophic keratitis.(Figure 1)3,4
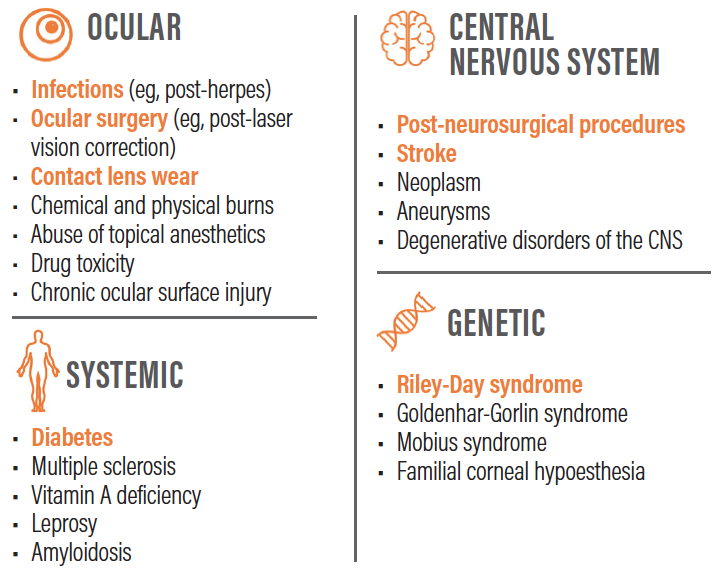
Figure 1. A whole host of etiologies ultimately lead to neurotrophic keratitis.3,4,5
Dr. Rapuano: In my experience, diabetes is a huge factor. I suspect neurotrophic keratitis potentially occurs frequently in patients who have diabetes. Diabetes in a patient’s medical history is a significant factor that leads me to suspect that a patient may have neurotrophic keratitis.5
Dr. Mah: If a patient with diabetes presents with signs of dry eye, I still perform my usual dry eye evaluation, but if the patient is asymptomatic, I immediately suspect neurotrophic keratitis.
Dr. Rapuano: I have found that patients with neurotrophic keratitis are often in the same age group as patients with dry eye, and they certainly can have both diseases.
Dr. Mah: When I see a patient whose chief complaint is blurry vision, absent foreign body sensation, and that patient has diabetes and a long history of chronic dry eyes, those factors are key when I’m considering neurotrophic keratitis, as opposed to dry eye.
Dr. Farid: We often talk about how symptoms and signs don’t necessarily correlate. There are patients who have minimal signs but are severely symptomatic, while patients with 4+ punctate keratitis and severe irregularity of the epithelium may report only fluctuating or blurry vision and no pain at all.5 The patient with significant signs and no symptoms of pain or discomfort has mild-stage neurotrophic keratitis. We may categorize those eyes as being in the spectrum of dry eye disease, but actually they have neurotrophic keratitis and are developing severe ocular surface disease secondarily.
Diagnosis Insights
Dr. Farid: Identifying neurotrophic keratitis early is important. What are you doing in your clinics to diagnose neurotrophic keratitis?
Dr. Mah: First, I confirm breakdown of the corneal epithelium. I look at the patient’s history, determine by testing that the patient has no corneal sensation, and then I make the diagnosis of neurotrophic keratitis.
Dr. Rapuano: Three key questions in the patient history will help identify people at risk for neurotrophic keratitis: Do you or have you ever had herpes simplex or zoster? Do you have diabetes? Have you ever had ocular surgery? Affirmative answers to any of these questions can capture patients who are at risk. Certainly, not all patients with these conditions have neurotrophic keratitis, but if they have one (or more) of these conditions, and a chronic ocular surface disease or a poorly healing epithelial defect, neurotrophic keratitis should be suspected.4,5
Sometimes, from a practical standpoint, we can’t make an accurate diagnosis at the first visit. The patient may already have received numbing drops for the IOP measurement before seeing me. In those cases, I write in my chart in large letters, ‘Next visit, zero drops until I see the patient,’ so the technician and the fellow know exactly what I want to test.
Dr. Farid: This brings up the importance of educating our staff, so they know not to numb the eye if we suspect neurotrophic keratitis. Often it does take two visits for me to confirm that diagnosis. If I think there may be underlying herpetic eye disease, I have the patient return for follow-up, and we make sure to not place proparacaine in the eye.
Dr. McDonald, how do you test corneal sensitivity?
Dr. McDonald: I perform a cotton wisp test. I lightly touch the cornea with a wisp of cotton from a cotton-tipped applicator to test the corneal reflex. This is not as quantitative as sensitivity testing with an esthesiometer, but cotton-tipped applicators are readily available in the office, and testing with a cotton wisp takes only a few seconds.4
Dr. Mah: Similar to a cotton wisp, I use a rolled-up corner of a tissue to test corneal sensitivity. I typically test just one spot in the center, rather than testing the quadrants. This enables me to grade sensitivity as present, decreased, or absent in patients with early disease. My suspicion is raised when I see a patient who has been using artificial tears, ointments, and various other treatments without any relief, so I perform corneal sensitivity testing on these patients as well.
Dr. Farid: Dr. Beckman, how important is documenting corneal sensitivity testing when you suspect neurotrophic keratitis?
Dr. Beckman: Corneal sensitivity testing is important to confirm a diagnosis of neurotrophic keratitis. If I expect to prescribe cenegermin or perform an intervention such as a tarsorrhaphy, I’m diligent as it confirms my diagnosis.
Dr. Rapuano: In my experience, sensitivity testing also helps convince patients that they need treatment, because I can prove it to them in the moment.
Dr. Beckman: When I’m treating patients with a history of diabetes, I relate their ocular condition to the fact that they are at risk for developing sores on their feet, but they may not feel them. In this case, they may have an ulcer on the cornea that they can’t feel. This analogy helps me educate patients on why they need treatment.
Dr. Farid: Once a patient has stage 2 or stage 3 neurotrophic keratitis and a large epithelial defect, the situation is urgent. As cornea specialists, we want to close that defect to avoid secondary infection. These patients will require significant chair time and frequent follow-up until the epithelial defect closes.
Dr. Mah: We must convey that urgency to patients. They know they have something wrong with their eye, but it doesn’t hurt, and it’s been there for weeks. Now, all of a sudden, we’re placing amniotic membrane on the eye and scheduling frequent follow-up visits. They are surprised and shocked.
Initiating Treatment
Dr. Farid: Figure 2 summarizes some common treatments for neurotrophic keratitis. Treatment choice can be influenced by neurotrophic keratitis stage and severity, as well as which treatments have been tried previously.4

Figure 2. Treatments for neurotrophic keratitis. 3,4,5
* The table is not an exhaustive list of all available treatment options.
Dr. Mah, how do you proceed if a patient has been using various medications before seeing you?
Dr. Mah: The referring doctor may have prescribed antibiotic or steroid eyedrops that contain benzalkonium chloride. Patients may also be using glaucoma medications that contain preservatives. Any of these therapies, which are intended to help patients, can potentially be toxic to the cornea.4 Consolidating medications or switching to nonpreserved agents is critical.
Dr. Rapuano: We also want to treat other conditions that are present, such as dry eye or blepharitis. Many patients have some degree of exposure keratopathy, which must be addressed, as well.
Dr. Beckman: As we’re ruling out comorbidities and washing out therapies, we’re sometimes faced with a dilemma. If we’re treating dry eye, perhaps with a preservative-free product such as cyclosporine ophthalmic emulsion, do we wash out everything? Suppose the medication is helping? Often, I have a patient continue using some of those medications. Sometimes, I stop everything. Switching patients to preservative-free glaucoma drops or performing selective laser trabeculoplasty can be useful. We have to make these decisions on a case-by-case basis, observe the results, and go to the next step.
Dr. Farid: Agreed. We can’t necessarily formulate an algorithm that will work for every patient.
Oxervate: Recombinant Human Nerve Growth Factor Targets the Root Pathogenesis of NK
Please see Important Safety Information about Oxervate
About Oxervate
Dr. Farid: Oxervate (cenegermin-bkbj ophthalmic solution) 0.002% (20 mcg/mL) is the first FDA-approved application of a recombinant human nerve growth factor (rhNGF) as a drug or treatment, and it is the first FDA-approved medication specifically indicated for neurotrophic keratitis.2 Cenegermin-bkbj, the active ingredient in Oxervate, is structurally identical to the human NGF protein found in ocular tissues.6
Two independent clinical trials enrolled the largest combined population of neurotrophic keratitis patients ever examined in randomized controlled trials. Patients in the trials administered Oxervate six times daily for 8 weeks.7,8 Based on these studies, Oxervate was granted orphan drug designation, fast-track status, breakthrough therapy designation, and was approved by the FDA under priority review in August 2018.1
At 8 weeks, 72% of patients who received Oxervate in the REPARO European trial had complete corneal healing (defined as 0 mm staining in the lesion area and no persistent staining in the rest of the cornea) compared to 33% of vehicle-treated patients.7 In the U.S. trial/NGF0214, 65% of patients had complete corneal healing compared to 17% of vehicle-treated patients.8
Importantly, 80% of patients who achieved complete corneal healing in the REPARO trial were still completely healed 48 weeks after one 8-week course of Oxervate.1,7
Dr. Rapuano: To me, the 48-week results are important. In my experience, many treatments can heal the cornea, but when the majority of corneal defects remain healed one year after treatment was stopped that is clinically relevant.
Dr. Farid: As for side effects, the most common is eye pain following instillation (16%),2 and I make it a point to explain to my patients that they may experience eye pain, which is a key part of my patient education.
Other adverse reactions, occurring in 1% to 10% of patients, were corneal deposits, foreign body sensation, ocular hyperemia, ocular inflammation, and tearing. There was no evidence of systemic absorption or immunogenicity in clinical trials.2
How Oxervate Fits Within Current Treatment Options
Dr. Farid: When a new therapy becomes available, I usually start using it for my most severe cases until I realize it works well in the earlier stage of the disease. Based on my experience with Oxervate, my threshold for when to start treatment of neurotrophic keratitis has become lower.
Dr. Beckman: I agree completely. I look at disease duration versus severity. If a patient has mild neurotrophic keratitis, I may try other therapies first, but if there’s no response, I prescribe Oxervate. The longer the duration of neurotrophic keratitis, even if fairly mild, the more likely I am to start Oxervate before the disease progresses in severity.
Dr. Mah: Having Oxervate has accelerated my treatment process. Instead of waiting for tears, gels, and ointments to work, I rapidly cycle through these therapies. If I don’t see a response within a couple of days with a bandage contact lens or an amniotic membrane, I move to Oxervate.
Dr. Farid: How do you manage earlier stage versus later stage neurotrophic keratitis? Would you consider prescribing Oxervate for a patient with mild neurotrophic keratitis?
Dr. Mah: Yes. I’ve done that, and I’ve found it works well.
Dr. Beckman: I think ‘early’ is a relative term. There’s early from the standpoint of where patients are on the spectrum, and there’s early from the standpoint of the treatments they’ve received. If I have a new patient who has been using cyclosporine ophthalmic emulsion for longstanding dry eye disease and there is no improvement, I may suspect this patient has something other than just dry eye. If I confirm the patient has neurotrophic keratitis, I might prescribe Oxervate immediately. If the patient is treatment-naive, I may first cycle through more conservative therapies for neurotrophic keratitis to see if the patient’s condition will resolve. In addition, if a patient has good visual potential and is at risk for scarring and permanent loss of vision, I might be even more aggressive.
Dr. Farid: The following cases demonstrate the real-world use of Oxervate in patients with neurotrophic keratitis.
The following cases are from the clinical practice of our panelists and did not participate in the clinical trial for Oxervate (cenegermin-bkbj ophthalmic solution) 0.002% (20 mcg/mL).
Case 1: Stage 2 Neurotrophic keratitis
Dr. Farid: My patient was a 75-year-old man with a history of bilateral LASIK and herpes zoster in the left eye. Those two underlying etiologies suggest neurotrophic keratitis.
One key factor to consider when evaluating a new patient in whom you suspect neurotrophic keratitis is his or her current topical medications, particularly those that could potentially harm the cornea, such as preserved glaucoma drops and topical antiviral agents.4 This patient was not using these medications, but any potentially toxic topical eyedrops should be identified in these cases.
Corneal sensitivity testing with a cotton wisp revealed complete anesthesia of the left eye. My clinical examination showed a large oval-shaped, stage 2 neurotrophic corneal epithelial defect (Figure 3).
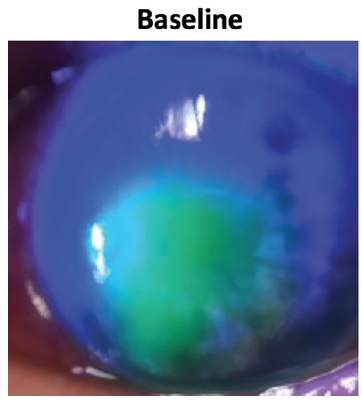
Figure 3. Case 1. Neurotrophic keratitis, stage 2, persisted despite multiple therapies. Image shows the cornea at baseline before starting Oxervate.
The referring doctor had prescribed antibiotic eyedrops and applied bandage contact lenses, as well as two rounds of AMT, but no improvement was noted.
I continued the antibiotic drops and aggressive preservative-free tears. I prescribed oral valacyclovir in case there was any recurrent herpetic etiology, and I started the patient on autologous serum. At the same time, I ordered Oxervate (cenegermin-bkbj ophthalmic solution) 0.002% (20 mcg/mL) from the specialty pharmacy.
We received the Oxervate in about 2 weeks, and the patient started using it six times a day for 8 weeks. During that time, I monitored his condition weekly or every other week. It’s important to continue treating inflammation and concomitant ocular surface disease such as blepharitis on a case-by-case, as-needed basis.
After 4 weeks of treatment with Oxervate, I saw considerable reduction in the size of the epithelial defect (Figure 4).
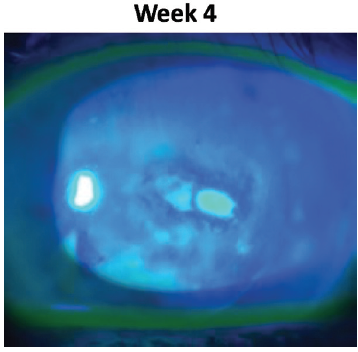
Figure 4. Case 1 after 4 weeks of Oxervate.
By the end of the 8-week course of treatment, not only was the epithelial defect healed, but the confluent superficial punctate keratitis and staining of the irregular epithelium around it was resolved (Figure 5). The patient continues to manage his ocular surface disease with preservative-free artificial tears and has been doing well for months.
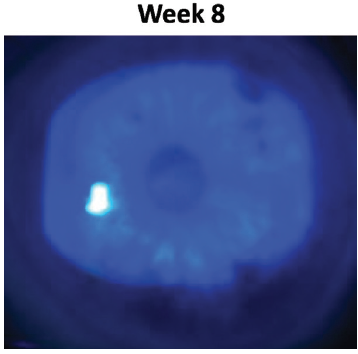
Figure 5. Case 1 after 8 weeks of Oxervate. The epithelial defect was healed and the confluent superficial punctate keratitis and staining of the irregular epithelium was resolved.
Case 2: Stage 3 Neurotrophic Keratitis
Dr. Rapuano: My patient was a 79-year-old man with a history of shingles in his left eye 20 years ago. He reported no major cornea problems since that time. He has undergone cataract surgery with IOL implantation; he has some post-herpetic neuralgia and atrial fibrillation, and he uses anti-coagulation medication.
The patient has had several retinal detachment repairs to his left eye, most recently 6 months ago, and his vision in this eye is chronically poor. Three weeks before I saw him, he developed conjunctivitis and was treated with antibiotic drops. One week before I saw him, he was being treated for a corneal abrasion with a bandage soft contact lens, which fell out after a few days. Then he came to see me.
Upon examination, I noted a 3 mm x 5.5 mm epithelial defect inferocentrally with heaped neurotrophic edges, some thinning, and no distinct infiltrate (Figure 6).
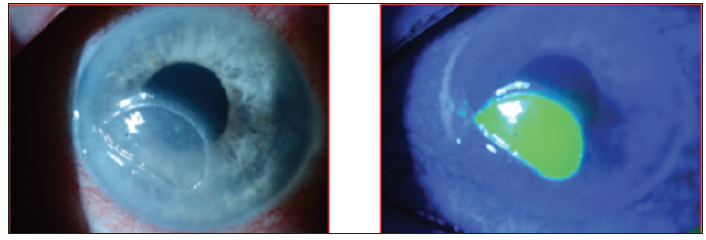
Figure 6. Case 2. A 79-year-old man with Stage 3 neurotrophic keratitis.
Corneal sensation, tested with a cotton wisp, was decreased in this eye compared to the other eye, and the diagnosis of stage 3 neurotrophic keratitis was confirmed. While the cornea did not appear to be infected, there was some thinning in the area of the corneal defect. This is always concerning, because we know that patients with neurotrophic keratitis have poor healing.
On the first day I saw the patient, I placed a self-retained double-thickness amniotic membrane and then treated the cornea with antibiotic ointment. I monitored the patient closely over the next 4 weeks, during which time he had another two amniotic membranes placed and also underwent a temporary tarsorrhaphy. Over this period, his stromal thickness was reduced by about 50%, and I was becoming concerned, so I felt it was best to schedule a multilayer AMT. I also performed a repeat partial tarsorrhaphy. At this time, I ordered Oxervate.
After a week and disintegration of the AMT, the patient started using the Oxervate medication. Figure 7 shows the cornea 2 weeks after starting Oxervate.
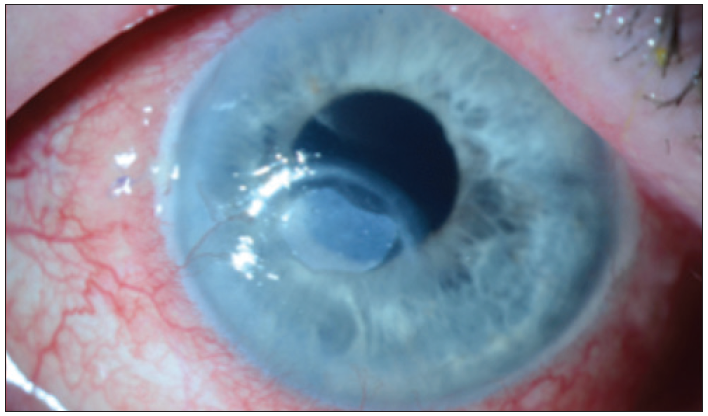
Figure 7. Case 2. Two weeks after starting Oxervate.
The patient completed the 8-week course of Oxervate, and 3 months later, the epithelial defect remained healed and the cornea was smoother, with very little punctate staining (Figure 8).
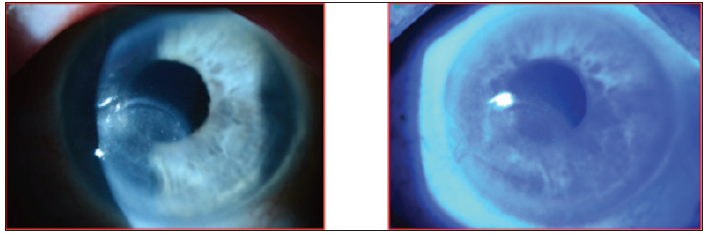
Figure 8. Case 2. Three months after completing an 8-week course of Oxervate.
I examined the patient recently, about 8 months after his Oxervate treatment, and he continues to do well, with minimal punctate staining.
Raising Awareness in the Eyecare Community
Dr. Farid: With the availability of Oxervate, a breakthrough therapy for neurotrophic keratitis, we are able to successfully treat this debilitating disease in a majority of patients.1 What key points would you share with our colleagues in the eyecare community to encourage them to integrate the diagnosis and treatment of neurotrophic keratitis into their practices?
Dr. Rapuano: I would use the example of keratoconus. While we could diagnose keratoconus in the past, it wasn’t until recently that we had an effective, FDA-approved treatment. Similarly, we now have an FDA-approved treatment for neurotrophic keratitis, something we didn’t have before. We have to shift the paradigm and diagnose neurotrophic keratitis early, because we have a treatment that is effective for treating it.
Connect to Care for Oxervate
Connect to Care is a set of comprehensive patient support programs developed by Dompé to facilitate access to Oxervate (cenegermin-bkbj ophthalmic solution) 0.002% (20 mcg/mL) for patients with neurotrophic keratitis. Dompé Connect to Care helps with the following:
- Verifying health insurance coverage for Oxervate
- Determining eligibility for financial assistance
- Supporting delivery and administration of Oxervate
Ask your Dompé representative for more information about Connect to Care or visit https://oxervate.com/hcp/patient-support/
Dr. McDonald: Those of us with experience using Oxervate for the treatment of neurotrophic keratitis can be resources for our colleagues who are just becoming familiar with it.
Dr. Beckman: It’s advantageous that eyecare practitioners are already looking for ocular surface disease. The initial step toward identifying neurotrophic keratitis and then integrating neurotrophic keratitis management into practice is getting practitioners to use stain to examine the cornea. Once they identify corneal disease, they need to go a step further and test corneal sensation.
Dr. Farid: In my experience, another patient population that warrants an evaluation for potential neurotrophic keratitis are those who have signs of dry eye but no symptoms.
Dr. McDonald: They are in the ‘stain with no pain’ category and could very likely have neurotrophic keratitis.
Dr. Farid: Yes. Those are the patients who are treated over and over again for dry eye, and they never heal well. Oxervate is a treatment option if they have a confirmed diagnosis of neurotrophic keratitis.
I would also emphasize to our optometry colleagues that they are the eyecare professionals most likely to see contact lens wearers—patients who are at risk for developing neurotrophic keratitis—and it is critical for them to recognize this early.
Dr. Rapuano: There may be some hesitation because of the cost of the therapy, but I have found that acquiring Oxervate (cenegermin-bkbj ophthalmic solution) 0.002% (20 mcg/mL) is a straightforward process. The majority of my patients have been able to receive the medication with a very low copay .
Dr. Farid: Treating neurotrophic keratitis is not just about helping patients feel better. Even in the milder stage, patients can potentially experience visual decline from untreated neurotrophic keratitis.4,5 The key is to lower the bar, learn to diagnose it, identify it early, and then treat it.
1. Drug approval package: OXERVATE (cenegermin-bkbj) [Internet]. U.S. Food and Drug Administration. 2018 Sep [cited 2018 Nov 13]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761094Orig1s000TOC.cfm.
2. Oxervate (cenegermin-bkbj) ophthalmic solution 0.002% (20 mcg/ml) [US package insert]. Boston, MA: Dompe U.S. Inc.; 2019.
3. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-579.
4. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107-131.
5. Mastropasqua L, Massaro-Giordano G, Nubile M, et al. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232:717-724.
6. Voelker R. New drug treats rare, debilitating neurotrophic keratitis. JAMA. 2018;320(13):1309.
7. Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332-1343.
8. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2019.
For additional information and full prescribing information, go to www.oxervate.com. To report SUSPECTED ADVERSE REACTIONS, contact Dompé U.S. Inc. at 1-833-366-7387 or FDA at 1-800-FDA-1088 or www.fda.gov./medwatch
Important Safety Information
Contact lenses should be removed before applying OXERVATE because the presence of a contact lens (either therapeutic or corrective) could theoretically limit the distribution of cenegermin-bkbj onto the area of the corneal lesion. Lenses may be reinserted 15 minutes after administration.
OXERVATE may cause mild to moderate eye discomfort such as eye pain during treatment. The patient should be advised to contact their doctor if a more serious eye reaction occurs.
The most common adverse reaction in clinical trials that occurred more frequently with OXERVATE was eye pain (16% of patients). Adverse reactions included corneal deposits, foreign body sensations in the eye, ocular hyperemia (enlarged blood vessels in the white of the eyes), swelling (inflammation) of the eye, and increase of tears (1-10% of patients).
WHAT IS OXERVATE™?
OXERVATE™ (cenegermin-bkbj) ophthalmic solution 0.002% is indicated for the treatment of neurotrophic keratitis.
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution for topical use in the eye: cenegermin-bkbj 0.002% (20 mcg/mL) is a clear, colorless solution in a multiple-dose vial.
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
Use With Contact Lenses
Contact lenses should be removed before applying OXERVATE because the presence of a contact lens (either therapeutic or corrective) could theoretically limit the distribution of cenegermin-bkbj onto the area of the corneal lesion. Lenses may be reinserted 15 minutes after administration.
Eye Discomfort
OXERVATE may cause mild to moderate eye discomfort such as eye pain during treatment. The patient should be advised to contact their doctor if a more serious eye reaction occurs.
ADVERSE REACTIONS
Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be compared directly to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In 2 clinical trials of patients with neurotrophic keratitis, a total of 101 patients received cenegermin-bkbj eye drops at 20 mcg/mL at a frequency of 6 times daily in the affected eye(s) for a duration of 8 weeks. The mean age of the population was 61 to 65 years of age (18 to 95).
The most common adverse reaction in clinical trials that occurred more frequently with OXERVATE was eye pain (16% of patients). Other adverse reactions included corneal deposits, foreign body sensation in the eye, ocular hyperemia (enlarged blood vessels in the white of the eye), swelling (inflammation) of the eye, and increase in tears (1%-10% of patients).
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no data from the use of OXERVATE in pregnant women to inform any drug-associated risks.
Administration of cenegermin-bkbj to pregnant rats or rabbits during the period of organogenesis did not produce adverse fetal effects at clinically relevant doses. In a pre- and postnatal development study, administration of cenegermin-bkbj to pregnant rats throughout gestation and lactation did not produce adverse effects in offspring at clinically relevant doses.
Data
Animal Data
In embryofetal development studies, daily subcutaneous administration of cenegermin-bkbj to pregnant rats and rabbits throughout the period of organogenesis produced a slight increase in postimplantation loss at doses greater than or equal to 42 mcg/kg/day (267 times the maximum recommended human ophthalmic dose [MRHOD]). A no-observed-adverse-effect level (NOAEL) was not established for postimplantation loss in either species. In rats, hydrocephaly and ureter anomalies were observed once each in fetuses at 267 mcg/kg/day (1709 times the MRHOD). In rabbits, cardiovascular malformations, including ventricular and atrial septal defects, enlarged heart, and aortic arch dilation, were observed once each in fetuses at 83 mcg/kg/day (534 times the MRHOD). No fetal malformations were observed in rats and rabbits at doses of 133 mcg/kg/day and 42 mcg/kg/day, respectively.
In a pre- and postnatal development study, daily subcutaneous administration of cenegermin-bkbj to pregnant rats during the period of organogenesis and lactation did not affect parturition and was not associated with adverse toxicity in offspring at doses up to 267 mcg/kg/day.
In parental rats and rabbits, an immunogenic response to cenegermin-bkbj was observed. Given that cenegermin-bkbj is a heterologous protein in animals, this response may not be relevant to humans.
Lactation
Risk Summary
There are no data on the presence of OXERVATE in human milk, the effects on breastfed infants, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OXERVATE and with any potential adverse effects on the breastfed infant.
Pediatric Use
The safety and effectiveness of OXERVATE have been established in the pediatric population. Use of OXERVATE in pediatric patients 2 years of age and older is supported by evidence from adequate and well-controlled trials of OXERVATE in adults with additional safety data in children.
Geriatric Use
Of the total number of subjects in clinical studies of OXERVATE, 43.5% were 65 years old and older. No overall differences in safety or effectiveness were observed between elderly and younger adult patients.
The risk information provided here is not comprehensive. To learn more, talk about OXERVATE with your health care provider or pharmacist.
US-OXE-2000003 01/20