The dry eye disease treatment landscape is fraught with complexities: although clinicians have a number of treatment options for patients, a major unmet need remains. Drops compose a large percentage of our prescription/Rx therapeutic options, but there are some therapies that are employing non-ocular routes of administration. As one such therapy entered the market in late 2021—Tyrvaya® (varenicline solution) nasal spray (Oyster Point Pharma)—a roundtable comprised of optometrists and ophthalmologists who commonly treat dry eye disease was convened to assess the challenges associated with treating dry eye disease as well as how therapies such as Tyrvaya® could affect patient outcomes.
This roundtable, which has been edited for brevity and clarity, will be published in four parts before a final, full compendium is available. In this first installment, we review the real-world treatment dynamics that have led to undertreated and unsatisfied patients and introduce a different approach to dry eye disease treatment.
A PRIMER ON TYRVAYA®
Mitch Ibach, OD, FAAO: One of the most recent therapies with a whole new way of generating natural tear film is Tyrvaya® (varenicline solution) nasal spray 0.03 mg which is approved by the US Food & Drug Administration to treat the signs and symptoms of dry eye disease (Figure 1).1 When I first heard of a nasal spray to treat dry eye disease, I was intrigued. I’ve found that dry eye patients are interested in a new route of administration after undergoing years of drop therapy.
Figure 1. Tyrvaya® (varenicline solution) nasal spray 0.03 mg was approved by the US FDA in October 2021 to treat the signs and symptoms of dry eye disease.
Marguerite B. McDonald, MD, FACS: Tyrvaya® piqued interest in a number of my patients, particularly because it didn’t require administering medication to their already irritated ocular surface. Many of my patients who are heavily reliant on an artificial tear (ie, taking it more than 4 to 6 times per day for relief) get excited about a non-drop alternative. Patients who use contacts lenses or those who wear eye make-up come to mind.*
Cecelia Koetting, OD, FAAO: I like that Tyrvaya® opens the door for a conversation about how dry eye disease is more complicated than just an irritated ocular surface. While the exact mechanism of action is unknown, Tyrvaya® is believed to activate the trigeminal parasympathetic pathway, resulting in basal tear film production. So, when patients ask how a nasal spray could be effective, I’m able to start a discussion about the importance of balanced tear film and the connection that exists between the nose and eye that innervates the production of basal tears (Figure 2). I find that framing Tyrvaya® as an activator of natural tear film that can harness the body’s power to produce natural tears is useful when educating patients about the biologic mechanisms at play with this therapy.
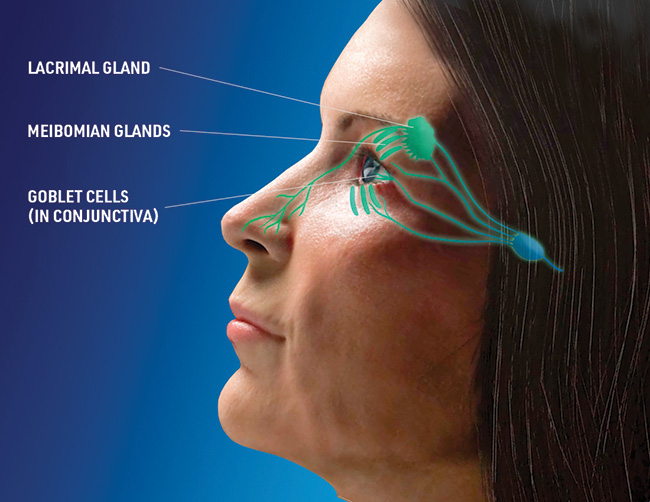
Figure 2. While the exact mechanism of action is unknown, Tyrvaya® is believed to activate the trigeminal parasympathetic pathway, resulting in basal tear film production.
Dr. McDonald: I, too, have found that Tyrvaya® helps me speak to patients about the overall dynamics of dry eye disease. I explain to my patients that 34% of basal tear production is stimulated by routine nasal respiration, and that we now have a means by which we can pharmacologically activate this biologic pathway.2 When framed in those terms, the connection between a nasal route of administration and the production of natural tear film makes more sense to many patients.
Inder Paul Singh, MD: Patients love that this product is basically telling their eyes to function as intended by activating the trigeminal parasympathetic pathway. If patients can produce natural tear film via activation of a biologic pathway, then they may be on their way to achieving tear film homeostasis. Clinicians may actually help patients address the root causes of their disease.
UNMET NEEDS IN DRY EYE DISEASE
Dr. McDonald: I often encounter patients with dry eye disease who have tried a number of over-the-counter artificial tears. By the time they reach my chair, they are frustrated. In some cases, patients have not been educated on two major issues: that dry eye disease is a chronic condition that can be treated but not cured, and that compliance with prescription medication is one of the keys to success. It’s our job to educate patients about these dynamics.
Dr. Ibach: As someone who practices at a tertiary referral center, I have had experiences similar to those described by Dr. McDonald. Another challenge I face is reconciling the disconnect between patient signs of dry eye disease and symptoms of dry eye disease. Sometimes they don’t add up. A patient may have plenty of signs of dry eye disease upon examination, but they report no symptoms. Alternatively, symptomatology may be well documented, but matching it with signs of disease is difficult.
Dr. Singh: As clinicians, we need to appreciate that signs and symptoms of dry eye disease are heterogenous across this large population. Some patients may have watery eyes, which they don’t associate with dry eye disease for obvious reasons. Other patients, particularly those who stare at screens for work, think that their fluctuating vision or eye fatigue is caused by computer usage, but don’t link those symptoms to dry eye disease.
Dr. Koetting: Explaining to patients that water streaming down their cheeks may be a sign of dry eye disease has its challenges. So, too, does gathering reliable patient history. Patients with long-term disease have tried so many different strategies that they sometimes fail to recall which treatments they’ve already undergone, which may complicate our efforts to treat them effectively. Trying an already failed therapy may delay the potential for relief, but skipping common tactics for addressing dry eye disease on the assumption that the patient had already tried it may lead clinicians to overlook less invasive solutions that may bring patients satisfaction.
Dr. Singh: I find a lack of reliable patient-reported history to also be true of patients who have ocular comorbidities, which makes sense given the number of drops they might use per day. Many of the patients in my practice use drops to control glaucoma, and the growing number of drops prescribed to them can be both confusing and cumbersome. Starting these patients on a new drop can be difficult.
Dr. McDonald: Sometimes when I start patients on a new drop, they ask if this drop will be used forever. I explain that although there is no cure for dry eye disease, we have many tools at our disposal to control it. Dry eye disease is a chronic, incurable disease that often requires the ongoing use of a prescription medication to manage.
Dr. Ibach: Given the various presentations and clinical observations relevant to dry eye disease, it’s useful to have an evidence-based definition of the condition. To that end, eye care providers often turn to the Tear Film & Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) definition of dry eye disease, which states that dry eye disease is a ‘multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.’3
That is a lot to digest, and I’m curious to hear from the panel what their initial reactions are when they encounter the TFOS DEWS II definition of dry eye disease.
Dr. McDonald: In the quest to manage patients, I think it is easy for clinicians to overlook the phrase TFOS DEWS II uses to open its definition: ‘loss of homeostasis of the tear film.’ This element of dry eye disease is not emphasized enough in the eye care community.
Dr. Singh: Patients present to us with symptoms that we can treat, and we might think of the loss of tear film homeostasis as the upstream factor that brought them to our clinic in the first place. Many of the current treatments employed by clinicians address the aqueous component of the tear film, but that doesn’t necessarily mean those treatments are helping to address tear film homeostasis.
Dr. Koetting: Elements of the TFOS DEWS II definition of dry eye disease concerning tear film instability and hyperosmolarity remind us that quantitative tools at our disposal need to be employed in order to accurately diagnose dry eye disease. If we point to objective metrics such as tear film break-up time, tear meniscus measurements, and osmolarity scoring, then we will be able to diagnose dry eye disease with greater confidence than if we were to rely on subjective data such as patient-reported symptoms.
Dr. McDonald: A commitment to comprehensive testing such as that described by Dr. Koetting is what defines a dry eye expert. Treating patients with signs of dry eye disease requires documentation and due diligence, and testing checks both of those boxes.
DECIDING ON TREATMENTS FOR DRY EYE DISEASE
Dr. Koetting: When a patient arrives in my clinic for a dry eye disease consultation, I find that an initial round of testing and a thorough slit-lamp examination provide a good baseline data set from which to work. I can determine if the patient’s disease is evaporative or aqueous deficient in nature, and I can understand which anatomic factors (meibomian gland status, blepharitis, etc.) might contribute to their disease.
Dr. Ibach: By the time a patient is in my chair at the referral center at which I practice, they’ve exhausted many options. I want to provide an alternative option that will provide symptom relief. This is especially true of my patients whose dry eye disease needs to be addressed before they can have cataract or refractive surgery.
Dr. McDonald: When do you begin to consider non-drop therapies for treating dry eye disease?
Dr. Singh: Drop therapy, even from over-the-counter options, can effectively address dry eye disease symptoms. Still, those treatments don’t address the disrupted homeostasis outlined in the TFOS DEWS II definition of dry eye disease. It’s great to have an alternative to drop therapy for patients that harnesses the power of a patient’s own natural tear film to address the signs and symptoms of dry eye disease.
Dr. Koetting: It’s all about the tear film to me. I would rather place the patient on a therapeutic route that will result in basal tear film production. For so many of these patients, the prospect of another drop is frustrating: they’ve tried various drops already, and they are still looking for something that might work for them.
Dr. Ibach: Patients may be familiar with the general safety profile of drop-based therapy, but often have questions about the expected side effects of Tyrvaya®. In general, I tell them that sneezing is likely to occur, as 82% of patients in the trials reported sneezing.1
Dr. Koetting: It’s important to remember that any level of sneezing was marked as an adverse event in the clinical trials, and 98% of those who experienced sneezing rated it as mild. Additionally, in the phase 3 trial, a majority of patients reported their sneezing resolved within one minute. In ONSET-2, 65% of sneezing resolved within one minute (159/244).4
Dr. Singh: I always want to make sure my patients are prepared for possible adverse events—that way, if they know about it ahead of time, an expectation was met. With that in mind, informing patients that cough, throat irritation, and instillation-site (nose) irritation were reported in 5% to 16% of patients in clinical trials is prudent.1
In our next installment, we’ll hear from a panel of eye care providers about their tactics for selecting patients who are suited for Tyrvaya® and will delve deeper into the mechanism of action for this novel approach to dry eye disease therapy.
TYRVAYA® IMPORTANT SAFETY INFORMATION
The most common adverse reaction reported in 82% of patients was sneezing. Events that were reported in 5-16% of patients were cough, throat irritation, and instillation-site (nose) irritation. Please see the full Prescribing Information at Tyrvaya-pro.com.
1. Tyrvaya. Prescribing Information. Oyster Point Pharma; 2021.
2. Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16(6):645-648.
3. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II reports executive summary. Ocul Surf. 2017;15(4):802-812.
4. Oyster Point Data on File.









