This edition of Global Products takes a look at the domestic scene. Guest Columnist Dr. Ayres discusses tools for the millennial ophthalmologist in conjunction with our Cover Focus “The Bigger Picture.”
—George O. Waring IV, MD
Every day, new ophthalmic devices are released to assist in medical decision making or improve surgical outcomes. Only a select few of these products, however, will actually change the way you approach a diagnostic or surgical dilemma. The trick is to know which of these devices is worth incorporating into your practice.
Ocular surface disease has an impact on all ophthalmic specialties as well as the general ophthalmologist. During the past several years, there has been an increasing emphasis on the diagnosis and treatment of dry eye disease (DED), and industry has responded with myriad new products. Four of the major entries into the ocular surface/external disease market include AdenoPlus (Nicox), InflammaDry (RPS, Inc.), tear film osmolarity detection (TearLab Osmolarity System; TearLab Corporation), and the LipiView Ocular Surface Interferometer with LipiFlow Thermal Pulsation System as treatment (TearScience).
POINT-OF-CARE DEVICES
Point-of-care devices have been a part of medicine for years. Recently, several point-of-care devices have become very useful in eye care. The first of these devices is the AdenoPlus. The device is an antigen detection system for adenoviral conjunctivitis. All that is necessary is a small sampling of the tear film along the lower palpebral conjunctiva, and within 10 minutes you have visual confirmation confirming or ruling out the presence of adenovirus. Accurately diagnosing an adenoviral infection is critical, as the infection spreads easily to other patients in the office, and signs and symptoms of bacterial, viral, and allergic conjunctivitis often overlap, making empirical diagnosis difficult. In fact, without the help of AdenoPlus, clinicians are correct in diagnosing adenovirus only 50% of the time (on a good day).1 By using the test, the office can set up a “Red Eye Protocol” where any patient with a red eye can be triaged, isolated, and tested by office staff before seeing the physician. The results of the test can be used to expedite patient care and minimize contact with other patients.
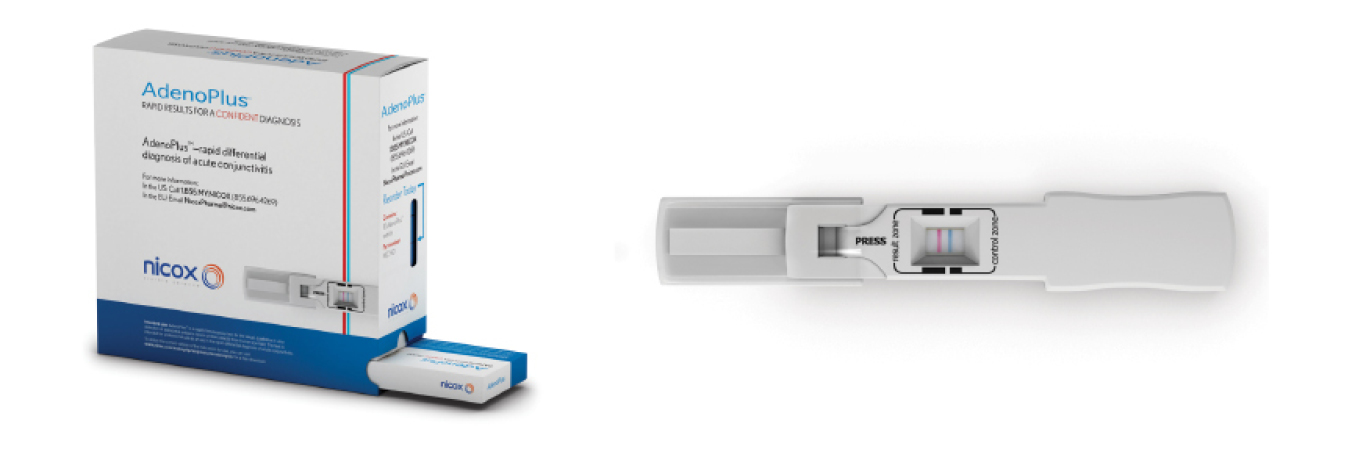
The AdenoPlus packaging and detector.
SJÖGREN SYNDROME
In the near future, Nicox will also be bringing to market a new dry eye panel for the detection of Sjögren syndrome. Sjögren syndrome is a highly underdiagnosed condition; current detection relies on identifying antibodies that tend to show up late in the disease process. Nicox’s new test, which includes a series of proprietary immune markers, will allow for significantly earlier and more specific detection of the condition. As ophthalmologists, we are often the first physicians to interact with and treat DED patients. Many Sjögren patients suffer from DED long before they are given a formal diagnosis. Being on the front lines of DED gives us an advantageous position in early detection of Sjögren syndrome. Early detection means early treatment, reducing long-term sequelae. The detection system will come to the office as a kit and work in conjunction with a lab. The clinician can choose send the patient to the lab for the test or perform the finger stick in the office and send the card to the lab; results can be obtained within 48 hours. The new panel can be complemented by InflammaDry, (RPS, Inc.) a point-of-care matrix metallopeptidase 9 (MMP-9) detector. Any patient with ocular surface complaints or clinical signs of DED can be screened with InflammaDry; if MMP-9 is elevated, treatment can be initiated with anti-inflammatory agents.
TEAR FILM TESTING
Along with looking for markers and antigens on the ocular surface, we now have the ability to look more closely at the qualities of the tear film. Tear film hyperosmolarity has been cited as the best marker for the diagnosis of DED2 and may be one of the earlier parameters to improve prior to clinical improvement.3 The Tearlab Osmolarity System samples 50 nL of tear and within seconds can read out the osmolarity. This number is not only useful in the diagnosis of DED, but also helps to monitor treatment. LipiView uses the concept of light interferometry to measure the lipid layer thickness of the tear film. This noncontact diagnostic test is an excellent way to measure the quality of the tear film, look at blink rate, and evaluate the patient for incomplete blink. If the lipid layer is low, indicating evaporative DED, patients may opt for treatment with the LipiFlow Thermal Pulsation System to accelerate healing. In a busy surgical practice, knowing the tear film indices can be beneficial, as they may indicate patients with asymptomatic borderline DED. These are the patients who will be more likely to decompensate after cataract surgery or have suboptimal vision with a multifocal IOL.
ABERROMETRY
Paying close attention to the ocular surface is critical in surgical patients but just as important, is making sure that biometric and topographic data are accurate, assuring correct IOL placement in both power and axis. As good as we think we are at cataract surgery, the fact is we are only about 65% accurate to within 1.00 D of our intended correction. To improve the accuracy of cataract surgery, intraoperative aberrometry is becoming commonplace. The ORA System (WaveTec Vision) is an add-on to the surgical microscope that allows intraoperative aberrometry (intraoperative refraction) to help verify IOL power and axis of toric IOLs. The most updated version of the ORA has what is called VerifEye. With the addition of VerifEye, the device provides real-time aberrometry, which speeds spherical power selection and toric alignment. Using intraoperative aberrometry, we can improve our accuracy to approximately 85% within 1.00 D of intended correction. An additional benefit is in post-LASIK, PRK, and RK patients. Use of the ORA is as accurate as and much easier than performing a battery of postrefractive calculations, in my experience.
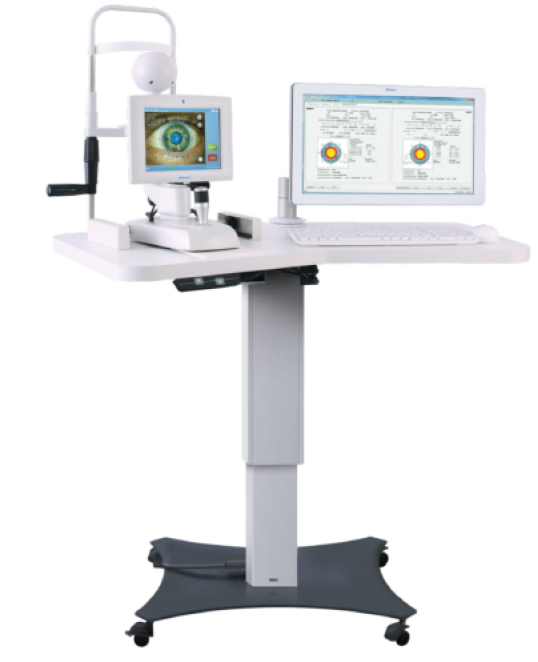
The Verion Reference Unit.
REFRACTIVE PREDICTABILITY
Another way to help improve refractive outcomes after cataract surgery is to improve accuracy and mapping of the surgical procedure. This is exactly what the Verion system (Alcon Laboratories, Inc.) is targeted to do. With this system, a “picture” is taken of the anterior segment along with keratometric data by a reference unit. Axial length is then added with optical or ultrasound units and surgical planning is performed including a plan for incision placement and reduction of astigmatism using AK/LRIs or a toric IOL. The Verion system then guides you through the surgery through a surgical overlay seen through the operative scope. The system will show you exactly where to place incisions, make relaxing incisions, and align and center IOLs, all with no need to mark the patient preoperatively. The goal is that with careful planning and exact transfer of the plan to the patient’s eye, we will also see an increase in surgical accuracy. Currently there is no intraoperative aberrometer in the Verion system, but I do think the two technologies could work together to help even further refine surgical accuracy.
CONCLUSION
New products and ideas are being brought to market daily. Often, it is hard to know what is going to be a fad and what is going to stand the test of time. In my opinion, the above listed products allow you to do, test, see, or treat something that you could not before and can change the way you approach a problem or surgery. I hope this helps, and best of luck in your own decision making.
1. O’Brien TP, Jeng BH, McDonald M, Raizman MB. Acute conjunctivitis: truth and misconceptions. Curr Med Res Opin. 2009;25(8):1953-1961.
2. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis of management of dry eye disease.
Am J Ophthalmology. 2011;151(5):792-798.
3. Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000-10008.



