In the way microstents and microinvasive glaucoma surgery are changing glaucoma treatment, a similar concept could be applied to cataract disassembly. One such approach is with the »miLoop (IanTech), a microinterventional device made of micro-thin, super-elastic, self-expanding nitinol filament technology. The miLoop has been designed to allow for the full-thickness fragmentation of the nucleus independent of phaco energy and uses centripetal, out-in nucleus disassembly to minimize capsular stress (Figure 1).

Figure 1 | The miLoop device is made of micro-thin super-elastic, self-expanding nitinol filament technology.
ENERGY REDUCTION
The ability to break up and remove the nucleus with less energy delivered to the corneal endothelium is of interest in cases in which the surgeon wants to minimize edema—for example, in patients with endothelial dystrophy or previous RK. In these situations, it is important to be able to assess the eye as soon after surgery as possible, so the less swelling the better. In general, reducing phaco energy has been shown to decrease corneal endothelial cell loss and potentially improve surgical outcomes.1-4
Femtosecond lasers increase the precision and accuracy of cataract surgery and reduce the amount of ultrasound energy required, but their cost is a large barrier to adoption. The laser has also made some steps of the procedure more difficult, such as cortical cleanup with irrigation and aspiration. With the miLoop, the ramp-up time is short; most surgeons report feeling comfortable using the device after four or five cases, as it is intuitive to implement.
IMPLICATIONS FOR THE DEVELOPING WORLD
According to IanTech, the miLoop is designed to work in harmony with conventional phaco techniques for a more synergistic cataract procedure. Although perhaps a niche device for now in the United States, one could imagine the problem-solvers of ophthalmology figuring out a way to use the technology to remove a cataract without phacoemulsification.
(Courtesy of Guillermo Amescua, MD)
Video 1 | Use of the miLoop in a patient with a black cataract.
The miLoop device has potentially major implications for the developing world, where cataracts can be denser and where phaco technology, if available, is not always optimal. Video 1 shows the device’s assistance in a black cataract case.
HOW IT’S DONE
Dr. Herren: I use a two-handed technique to insert the miLoop (Video 2). With the device in the eye, I extend the loop to its full length and then rotate it into position. Before I draw the miLoop back with my right hand, I use my second instrument—a Connor Wand (Rhein Medical)—in my left hand at the distal end of the loop to keep the lens from tilting up and out of the bag.
(Courtesy of Michael Greenwood, MD)
Video 2 | Two-handed technique used to insert the miLoop.
I create two hemi-nuclei, and then, with the device still extended, I spin the nucleus inside the loop and repeat to create four quadrants (Figures 2 to 4). I bring each piece up one at a time, typically using only about one-third of the amount of phaco energy used in a standard case. The miLoop allows for more manual chopping.
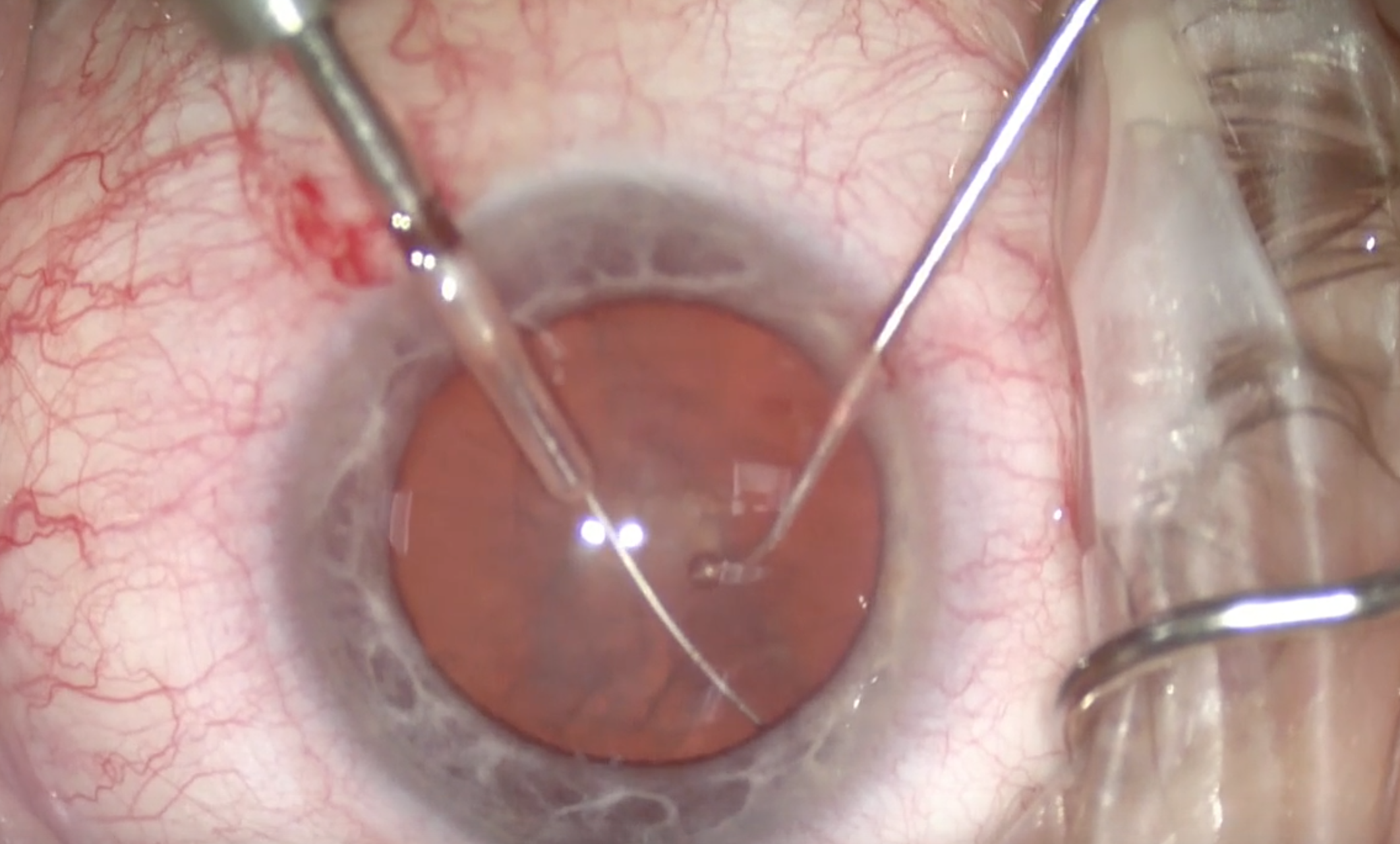
(Courtesy William F. Wiley, MD)
Figure 2 | The miLoop is extended and ensnares the lens.
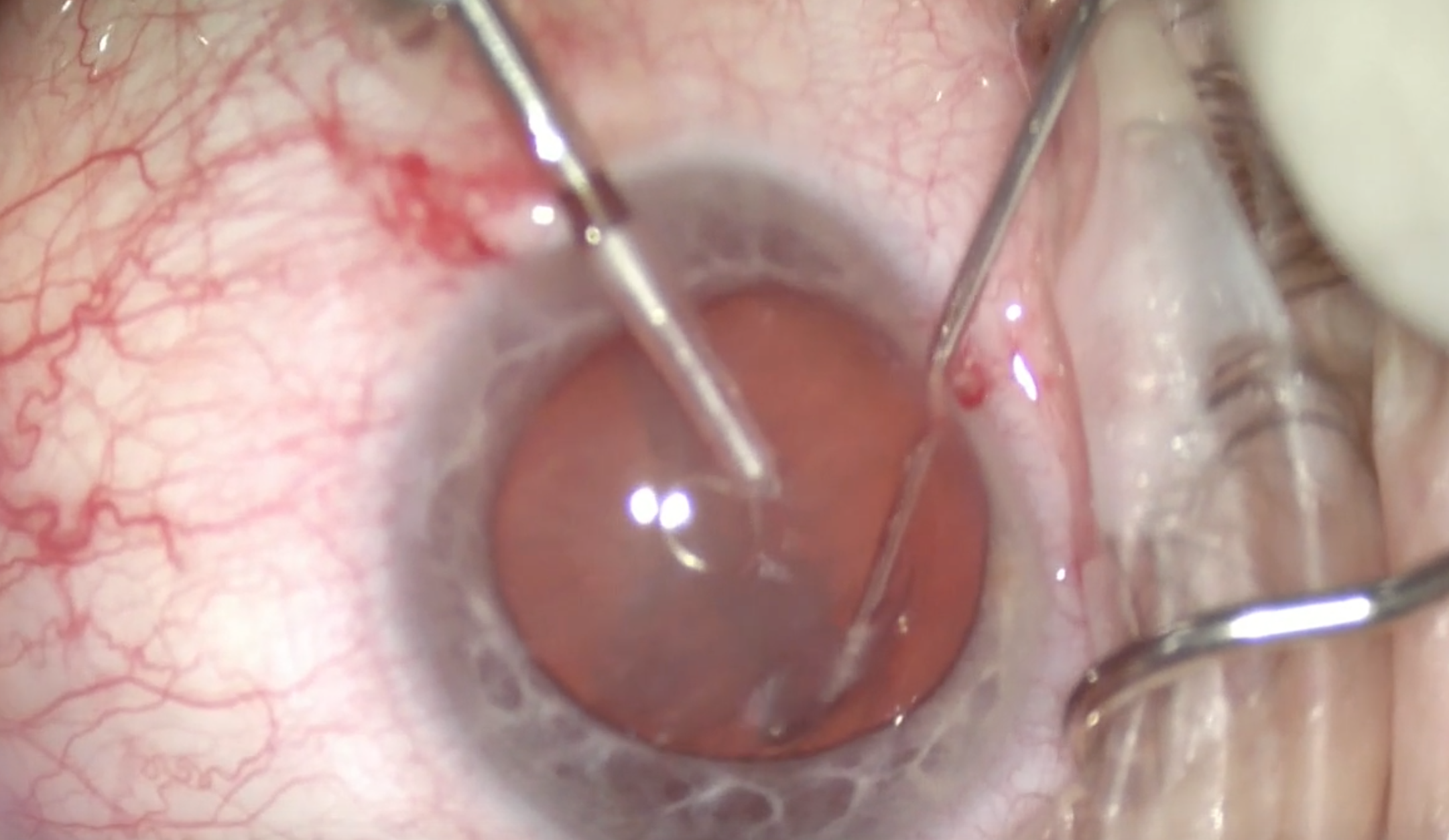
(Courtesy William F. Wiley, MD)
Figure 3 & 4 | The device is contracted to chop the lens.
The miLoop could be compared with a prechopper of sorts, but better. A prechopper works from the inside out, which puts stress on the zonules. Instead, the miLoop moves from the outside in. It also sweeps the cortex and is effective in dense nuclei, unlike a prechopper.
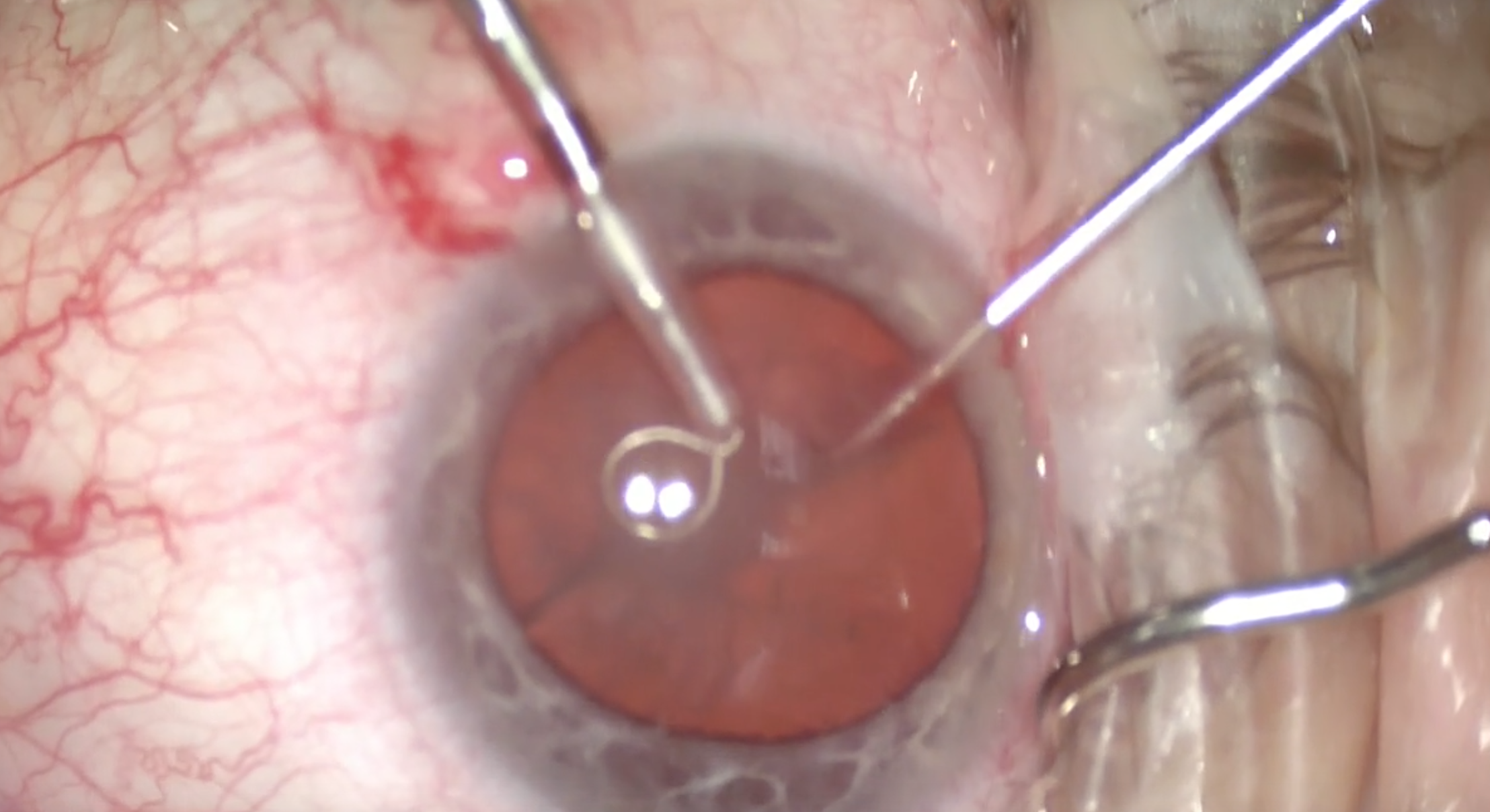
Dr. Amescua: My technique has been changing as I get more comfortable with the device. I find the miLoop easy to use in moderate-density cataracts of grades 2 or 3. In very dense nuclei (Figure 5), I use a second instrument—my viscoelastic cannula or chopper—in my nondominant hand to push the lens down. I make sure to apply pressure when I lasso the nucleus in the final stage of its cutting to keep it in place as the miLoop is coming out. With the second instrument, I spin the lens and cut again to obtain four quadrants.
(Courtesy William F. Wiley, MD)
Figure 5 | Black cataract removal made easier with the miLoop.
The miLoop works to mechanically remove the cortex, sweeping the peripheral capsule (Figure 6). With the femtosecond laser, it can be harder to obtain purchase because it does not create a flap of cortex to grasp.
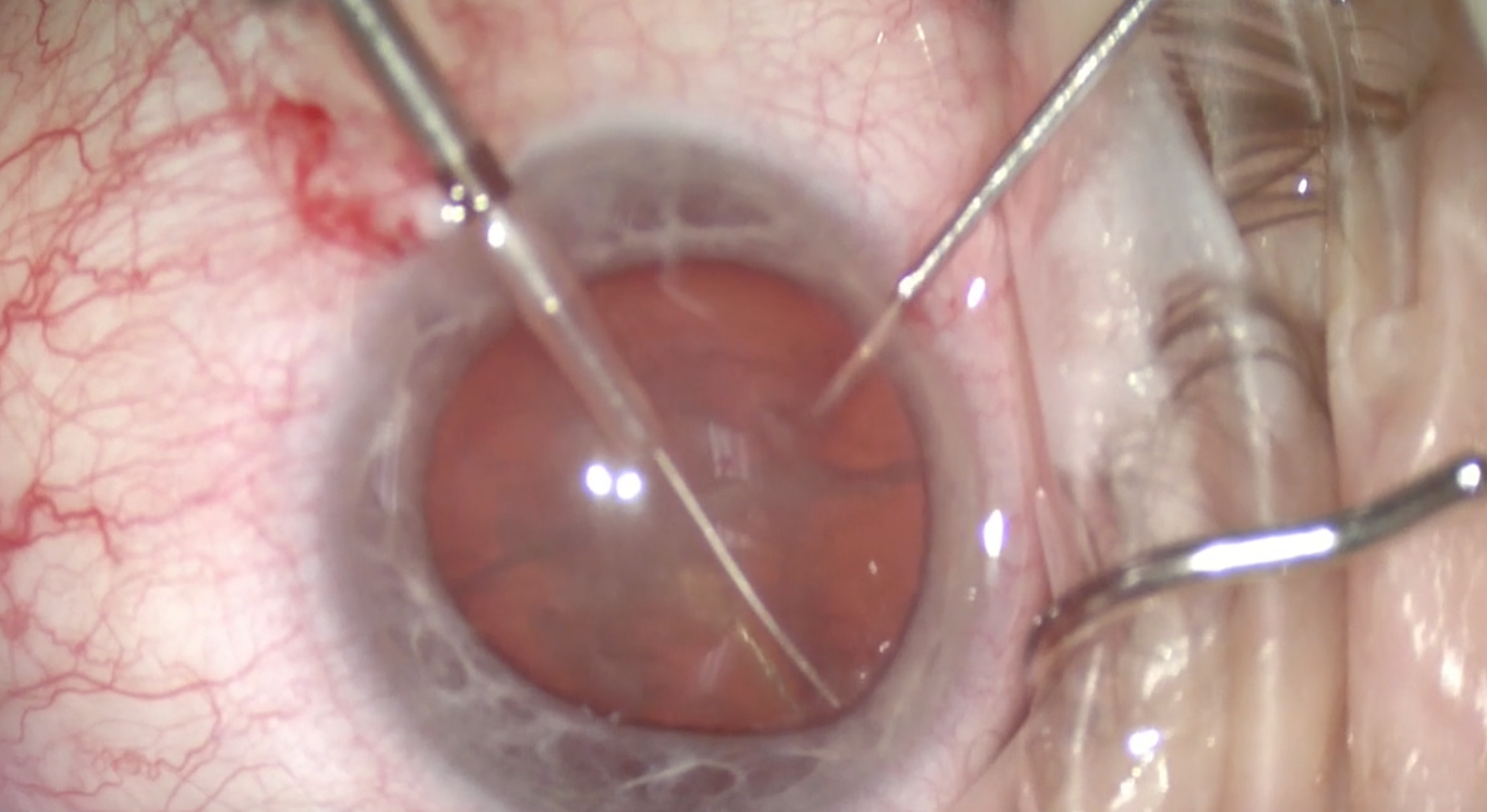
(Courtesy William F. Wiley, MD)
Figure 6 | The miLoop sweeps the cortex from the posterior capsule.
CONCLUSION
Having the option to reach for a tool like the miLoop during cataract surgery allows surgeons to have more confidence in cases in which reducing the amount of edema is crucial. The device uses nitinol filament technology to encircle the nucleus and slice through even very dense cataracts. Performing this fragmentation procedure through a small incision under viscoelastic is safe and easy, and it appears to be gentle on the ocular structures, enabling a more simplified and less-traumatic phaco process.
1. Pirazzoli B, D’Eliseo D, Ziosi M, Acciarri R. Effects of phacoemulsification time on the corneal endothelium using phacofracture and phaco chop techniques. J Cataract Refract Surg. 1996;22(7):967-969.
2. Kreisler KR, Mortenson SW, Mamalis N. Endothelial cell loss following “modern” phacoemulsification by a senior resident. Ophthalmic Surg. 1992;23(3):158-160.
3. Colvard DM, Kratz RP, Mazzocco TR, Davidson B. Endothelial cell loss following phacoemulsification in the pupillary plane. J Am Intraocul Implant Soc. 1981;7(4):334-336.
4. Hayashi K, Hayashi H, Hakao F, Kayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22(8):1079-1084.




