Despite recent decreases in reimbursement, it remains an exciting time to practice ophthalmology thanks to innovation in pharmaceuticals for the anterior and posterior segments, novel drug delivery systems, ocular imaging, and minimally invasive anterior and posterior segment surgery. Furthermore, the drug development pipeline is robust and bullish for anterior segment disease. Here is a review of many promising medications in development.
CORNEA
Dry Eye Therapy
Eleven Biotherapeutics is developing EBI-005 for the treatment of dry eye disease (DED) and severe allergic conjunctivitis by targeting the Interleukin 1 receptor. The company is pioneering the creation of a topical rather than injectable Interleukin 1 receptor blocker.1 According to Medical Director Michael Goldstein, MD, MBA (written communication, May 2013), EBI-005 will continue phase 2 trials later this year. Per the company’s press release on June 28, 2013, the drug has achieved excellent results thus far in a phase 1b/2a study. Patients reportedly experienced a statistically significant improvement in DED, and EBI-005 was safe and well tolerated.
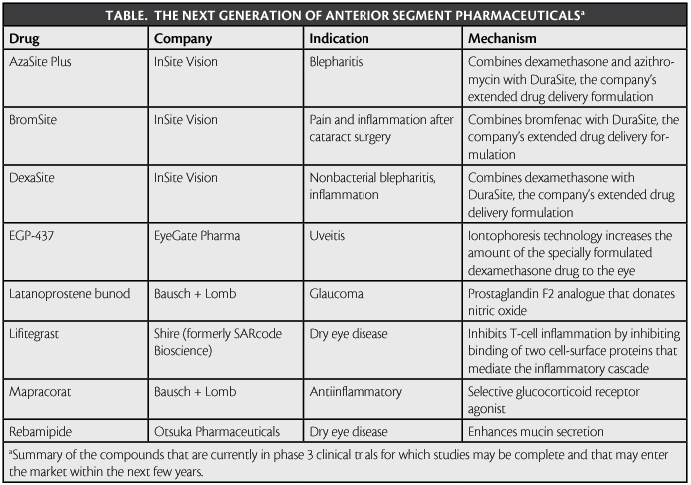
Rebamipide ophthalmic suspension (OPC-12759; Otsuka Pharmaceuticals) is currently in phase 3 trials in the United States.2 The drug enhances mucin secretion and is currently approved for the treatment of DED in Japan.2 Per the company’s press release on May 6, 2011, the drug has been superior to sodium hyaluronate.
According to a press release from R-Tech Ueno on April 26, 2013, the company received FDA approval to initiate phase 1 and 2 clinical trials of its compound RU-101, which contains recombinant human serum albumin and has downstream effects on tear production.
Lifitegrast (SAR 1118) prevents protein from binding to the cell, thereby inhibiting T-cell activation and the inflammatory cascade.3 According to a press release from SARcode Bioscience on October 23, 2012, the first phase 3 trial, which concluded in May 2012, demonstrated the drug’s superiority to placebo without serious adverse effects.4 Per the same announcement, a safety study (SONATA) is underway, and a second phase 3 trial (OPUS-2) is recruiting patients.5,6 Shire recently acquired SARcode Bioscience, along with global rights for lifitegrast. Shire anticipates launching the drug in the United States as early as 2016.7
Anti-infective Agents
Two companies are conducting trials of drugs for use against adenoviral conjunctivitis. Foresight Biotherapeutics is developing FST-100, which is the subject of an ongoing phase 2 study.8 FST-100 uses the company’s proprietary platform of povidone-iodine combined with dexamethasone.9
According to a press release from NovaBay Pharmaceuticals on April 8, 2013, the company began a phase 2b trial of Auriclosene (NVC-422), which enhances oxidative burst to destroy microbes.10 The trial will take place in multiple countries; the study just enrolled its first Brazilian patient in April 2013.
Antiinflammatory Drugs Mapracorat is under development by Bausch + Lomb. This selective glucocorticoid receptor agonist blocks the production of inflammatory mediators such as cytokines and prostaglandin.11 Two ongoing phase 3 trials are studying Mapracorat’s efficacy at treating pain and inflammation after cataract surgery.12,13
According to a press release on April 9, 2013, EyeGate Pharma is developing EGP-437, a corticosteroid that showed results equal to those of prednisolone acetate 1% for treating uveitis in phase 3 studies. The same compound is in phase 2 trials for the treatment of DED and in phase 1 trials for the treatment of scleritis.14
InSite Vision has three compounds with antiinflammatory indications: ISV-502 (AzaSite Plus) to treat blepharitis, ISV-303 (BromSite) to treat pain and inflammation after cataract surgery, and ISV-305 (DexaSite) to treat nonbacterial blepharitis and ocular inflammation. 15 According to a press release on March 25, 2013, the company received special permission to launch the Dual Ophthalmic Agents Used in Blepharitis (DOUBLE) study for the simultaneous phase 3 evaluation of AzaSite Plus and DexaSite in the same trial. Results are expected by mid-2013. Per the company’s March 19, 2013, press release, BromSite has completed a phase 3 clinical trial, which showed that the drug is efficacious and superior to first-line treatment for postoperative pain and inflammation. InSite Vision announced on March 18, 2013, that it will begin another phase 3 trial to prepare for approval in the United States and Europe.
GLAUCOMA
Rho-associated Protein Kinase Inhibitors
A promising new class of drugs under development is called the Rho-associated protein kinase (ROCK) inhibitors. 16 They modify the actin cytoskeleton to increase aqueous outflow, thereby lowering IOP.17 Additionally, these drugs have been found to enhance blood flow to the optic nerve.16 Multiple drugs in this category are in the pipeline.
Aerie Pharmaceuticals is developing AR-13324, AR-12286, and PG286 (combination therapy with AR-12286 and travoprost). All three are in phase 2 studies, and in November 2012, the company reported positive phase 2a results for both AR-13324 and AR-12286.1,18 In February 2013, Aerie began a study to compare AR-12286 with travoprost (PG286), AR-12286 alone, and travoprost alone.19
Altheos, Inc., is developing ATS907 and ATS8535. The latter was recently selected as a candidate for further study.20 After achieving promising results in animal models, phase 2a trials of ATS907 began in early 2012.20 The study is ongoing (recruitment of participants complete), but no results have been reported yet.21
A phase 2 study of Amakem’s drug candidate AMA0076 began in the fall of 2012 and is currently recruiting candidates.22 According to the company’s press release on September 27, 2012, preclinical studies demonstrated superior IOP-lowering results with this agent versus latanoprost but without hyperemia—a major side effect of other ROCK inhibitors in development that limits dose escalation in trials.
Prostaglandin Analogue
On January 29, 2013, Bausch + Lomb announced that it is developing latanoprostene bunod (BOL-303259-X). The mechanism by which this drug lowers IOP is unique compared to other medications on the market and the ROCK inhibitors: latanoprostene bunod is a prostaglandin F2 analogue that works by donating nitric oxide. Phase 2b results were promising, showing that the agent consistently lowered IOP to a greater degree than latanoprost 0.005% (Xalatan; Pfizer Inc.) without increased adverse effects.23 Latanoprostene bunod entered phase 3 trials in January 2013.
CONCLUSION
The future of eye care therapeutics is bright. The drugs in development are being designed to address the growing clinical needs of patients while minimizing side effects.
1. Helzner G. Bright new ideas in glaucoma treatment. Ophthalmology Management. January 1, 2013.
https://www. ophthalmologymanagement.com/articleviewer.aspx?articleID=107874. Accessed June 11, 2013.
2. Osterweil N. Dry eye symptoms eased by rebamipide, a mucin secretagogue. Medscape Medical News. April 4, 2013.
https://www.medscape.com/viewarticle/781948. Accessed June 11, 2013.
3. Our pipeline. Shire website. https://www.shire.com/shireplc/en/rd/pipeline. Accessed June 19, 2013.
4. Safety and efficacy study of SAR 1118 to treat dry eye (OPUS-1). https://clinicaltrials.gov/ct2/show/NCT0142149 8?term=sarcode&rank=3. Updated May 28, 2012. Accessed June 11, 2013.
5. Safety study of lifitegrast to treat dry eye (SONATA). https://clinicaltrials.gov/ct2/show/NCT01636206?term=sar code&rank=2. Updated February 27, 2013. Accessed June 11, 2013.
6. A phase 3 study to evaluate the efficacy of lifitegrast in subjects with dry eye. https://clinicaltrials.gov/ct2/show/ NCT01743729?term=sarcode&rank=1. Updated June 7, 2013. Accessed June 11, 2013.
7. Shire to acquire SARcode Bioscience, expands presence in ophthalmology. Shire website. https://www.shire. com/shireplc/en/media/shirenews?id=749. Published March 25, 2013. Accessed June 19, 2013.
8. FST-100 in the treatment of acute adenoviral conjunctivitis. https://clinicaltrials.gov/ct2/show/NCT01470664?ter m=adenoviral+conjunctivitis&rank=2. Updated December 21, 2012. Accessed June 11, 2013.
9. Technology. Foresight Biotherapeutics website. https://www.foresightbiotherapeutics.com/pub/platforms.html. Accessed June 11, 2013.
10. Wang L, Belisle B, Bassiri M, et al. Chemical characterization and biological properties of NVC-422, a novel, stable N-chlorotaurine analog. Antimicrob Agents Chemother. 2011;55(6):2688-2692. https://www.ncbi.nlm.nih.gov/pubmed/21422212.
11. Vollmer TR, Stockhausen A, Zhang JZ. Anti-inflammatory effects of mapracorat, a novel selective glucocorticoid receptor agonist, is partially mediated by MAP kinase phosphatase-1 (MKP-1). J Biol Chem. 2012;287(42):35212-35221. https://www.ncbi.nlm.nih.gov/pubmed/22898817.
12. Mapracorat ophthalmic suspension, 3% for the treatment of ocular inflammation and pain following cataract surgery.
https://clinicaltrials.gov/ct2/show/NCT01591161?term=mapracorat&rank=3. Updated May 14, 2013. Accessed June 11, 2013.
13. Mapracorat ophthalmic suspension, 3% for the treatment of ocular inflammation and pain following cataract surgery.
https://clinicaltrials.gov/ct2/show/NCT01591655?term=mapracorat&rank=4. Updated May 14, 2013. Accessed June 11, 2013.
14. Pipeline. EyeGate Pharma website. https://www.eyegatepharma.com/therapeutics/pipeline5.html. Accessed June 11, 2013.
15. Product pipeline. InSite Vision website. https://www.insitevision.com/product_pipeline. Accessed June 11, 2013.
16. Wirostko B. New class of glaucoma drugs on the horizon. Glaucoma Research Foundation website. May 2012.
https://www.glaucoma.org/treatment/new-class-of-glaucoma-drugs-on-the-horizon.php. Accessed June 11, 2013.
17. Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11(7):541-559. https://www.ncbi.nlm.nih.gov/pubmed/22699774.
18. Portfolio of glaucoma products. Aerie Pharmaceuticals, Inc., website. https://www.aeriepharma.com/aerietech. html. Accessed June 11, 2013.
19. A study assessing pg286 ophthalmic solution, 0.5% compared to its individual components for 28 days.
https:// clinicaltrials.gov/ct2/show/NCT01789736. Updated April 8, 2013. Accessed June 11, 2013.
20. Pipeline. Altheos website. https://altheos.com/Pipeline.html. Accessed June 11, 2013.
21. Study of the safety and efficacy of ats907 in subjects with primary open angle glaucoma (POAG) and ocular hypertension.
https://clinicaltrials.gov/ct2/show/NCT01520116. Updated October 30, 2012. Accessed June 11, 2013.
22. Multiple dose-escalation study of AMA0076 in patients with ocular hypertension or primary open-angle glaucoma.
https://clinicaltrials.gov/ct2/show/NCT01693315?term=amakem&rank=1. Updated November 23, 2012. Accessed June 11, 2013.
23. Boughton B. New glaucoma drug may be more effective than latanoprost. March 28, 2013. Medscape.
https:// www.medscape.com/viewarticle/781624. Accessed June 11, 2013.