A number of promising technologies are either entering into or emerging from the US development and regulatory pipeline. These technologies range from therapeutics to diagnostics, all playing an important role in advanced ocular surgery. However, the onerous regulatory process in the United States has caused the innovation cycle to increasingly move off shore. In response to the overregulation by the FDA, we’ve been forced to develop innovative ways to innovate in this culture, and it’s very different than the traditional pathway. A lot of what we do now isn’t just about making the widget safe and efficacious, but doing it most efficiently in the current regulatory environment.
Companies can more easily conduct clinical trials and plan commercial launches outside the United States where regulatory approval comes much sooner in the development process. Furthermore, with commercialization efforts, companies can generate revenue to offset the astronomical research and development costs required for an FDA clinical trial. Some companies are forgoing US commercialization altogether because the costs involved limit the potential for profitability. The United States will continue to play a vital role as regulatory approval here often leads to a level of acceptance by the global scientific community. This situation presents a bit of a conundrum for companies that choose to ignore the US market because it’s not worth going through the FDA process. With that said, I think we would all agree that the trajectory of overregulation by the FDA is not sustainable.
Early in my career, I found it necessary to travel outside the confines of the “regulatory boarders” of the United States to be able to be involved with new surgical devices. These valuable experiences allowed me to use and evaluate surgical devices not approved in the United Sates and help develop them as well. I have been an early adopter of many new technologies in the United States, including the first high-repetition rate Alcon EX500 excimer laser in the country and the first OptiMedica Catalys femtosecond laser in the state. Being an early adopter requires a commitment to investment in new technology and/or a connection with industry to allow one to help develop devices either through a clinical trial or medical advisory capacity. For bench research, I founded an ocular biomechanics laboratory within the department of bioengineering where we carry out translational research. These types of translational research labs will pop up more and more in the United States in both academic centers and increasingly so in the private sector, where they’ll be run efficiently like businesses.
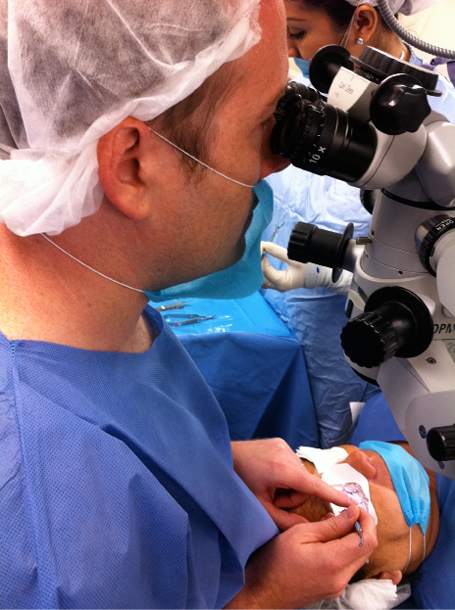
Figure 1. The author implanting corneal inlays (not approved in the United States).
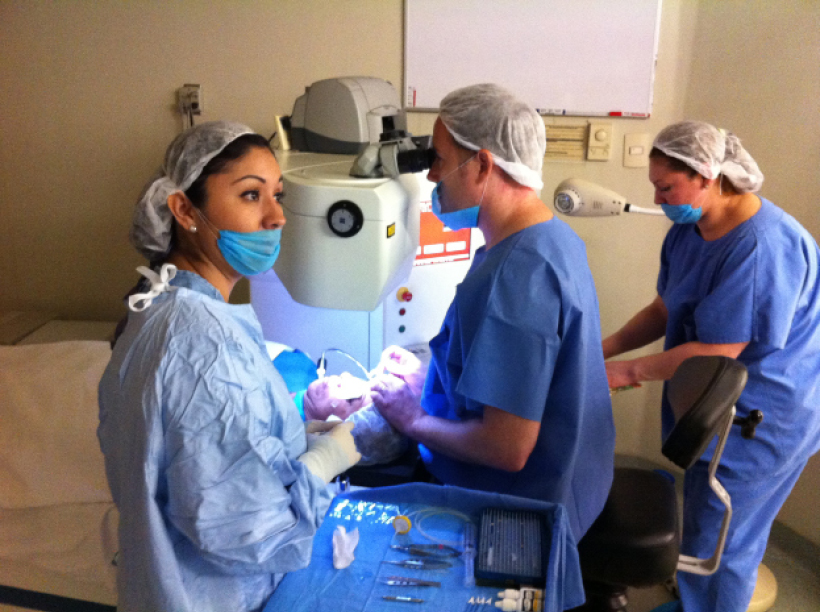
Figure 2. The author implanting corneal inlays in Mexico.
Figure 3. The author implanting corneal inlays during live surgery in Spain.

Figure 4. A team of surgeons after successfully completing a new surgical technique in Japan.
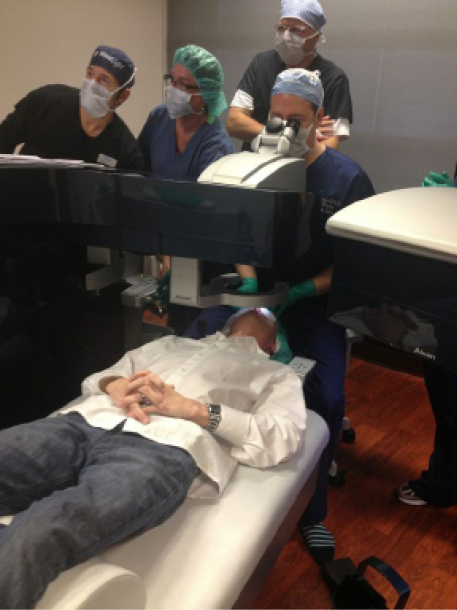
Figure 5. The first high-repetition excimer laser used in the United States.

Figure 6. The first Optimedica Catalys use in South Carolina.
Globalization of medical innovation requires cloud-based data exchange. Accelerated Vision has formed a company around this need and is poised to revolutionize global data transfer in a secure and instantaneous way. This data management and analytics company will more readily allow the simultaneous ability to run offshore trials and data gathering through commercialization efforts. In addition, it is positioned to define how clinical trials are run in the United States. Ophthalmic innovation microincubators are also becoming more commonplace. Cirle, a medical technology incubator, based in Miami, and founded by Richard Awdeh, MD, is one example of a partnership between industry (Bausch + Lomb) and academia (Bascom Palmer). Cirle is currently building a small, high-performance team of biomedical and chemical engineers, along with staff scientists, to pursue research and product development initiatives. Lastly, private equity firms such as Strathspey Crown have realized the need and opportunity to focus exclusively on the self-pay, innovation aspects of the med-device industry. Strathspey Crown positions itself as a lifestyle health care-focused growth equity firm that uniquely partners with specialty physicians to build highly innovative lifestyle health care technologies and services that provide life enhancement and wellness to patients worldwide.
Innovation is such a significant part of our specialty. Recently, groups such as the American-European Congress of Ophthalmic Surgery and the American Academy of Ophthalmology are finding ways to collaborate with the FDA to explore industry growth in a sustainable and responsible manner. These are great examples to follow and to become involved with. In many ways, the mission statement of MillennialEYE represents this spirit of innovation and future growth, for you are the future of our industry.

