Demand drives supply. The large and growing population of glaucoma patients has been driving the development of a number of new treatments, many providing an alternative to endless medications or complicated filtering surgeries. Such is the case with the formation of Glaukos Corporation: the result of collaboration between a life sciences entrepreneur, an ophthalmologist, and an aeronautics engineer, which began with the desire to help a family member diagnosed with glaucoma at a young age. Within a year of being founded, Glaukos invented a microbypass stent designed to reestablish physiologic outflow of aqueous and reduce IOP and performed the first implant in a human.
FIRST AB INTERNO GLAUCOMA IMPLANT
The iStent Trabecular Micro Bypass was granted FDA approval in June of 2012 as the first ever ab interno glaucoma implant to be approved in the United States. The iStent is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild to moderate open‐ angle glaucoma currently treated with ocular hypotensive medication. The microstent creates a patent bypass, allowing aqueous to flow from the anterior chamber into Schlemm canal, restoring the natural outflow pathway of the eye. The US pivotal trial showed that a single iStent in combination with cataract surgery lowered IOP by 33%, effectively replacing at least one medication.1
The approval of the iStent marks the beginning of a category of microinvasive glaucoma surgery (MIGS), which includes nonpenetrating procedures that mitigate several potential risks in comparison to traditional glaucoma surgery and require significantly less postoperative care. MIGS procedures can occur in Schlemm canal, the suprachoroidal space, or the subconjunctival space, and they share the following characteristics: cause minimal trauma, are effective at lowering IOP, have a favorable safety profile, and a rapid recovery period.
MIGS procedures not only possess the potential to lower IOP and reduce dependence on medications, but they are also highly synergistic with cataract surgery. The least ideal scenario after placing a premium IOL in a patient with glaucoma is to perform a filtering procedure that diminishes the success of the refractive outcome.
A PORTFOLIO OF MICRO-BYPASS STENTS
The iStent is just the first approval in Glaukos’ portfolio of micro-bypass stents aimed at improving the safety and efficacy outcomes of surgical implant procedures. Following the first-generation iStent are the iStent inject and the iStent supra. The second-generation device, iStent inject, is also a trabecular bypass device that comes preloaded with two stents in an easy-to-use injector. The next slated device is the iStent supra, a polyethersulfone stent that is inserted into the suprachoroidal space in cases where additional IOP reduction is needed beyond stenting of the conventional outflow system.

iStent inject.
The science behind MIGS continues to grow, as Glaukos is currently conducting two ongoing US investigational device exemption trials, three postmarket studies, and 18 international trials looking to further strengthen the already substantiated data on the benefits of the first-generation microbypass stent. Currently, the iStent is commercialized in nine countries including the United States, Canada, Germany, United Kingdom, France, Italy, Spain, Portugal, and New Zealand.
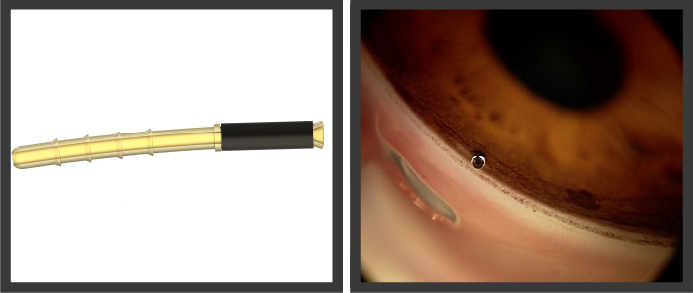
iStent supra.
FUTURE DIRECTIONS
In the future, the micro-stent technologies may be used as a foundational therapy to treat the widest range of open-angle glaucoma, with advances in delivery devices that will facilitate and expedite the procedure, as well as enable surgeons to intervene at the earliest stages of open-angle glaucoma. Targeted stent placement within Schlemm canal may further optimize the effectiveness of micro-bypass stent therapy.
Glaukos is a privately held, well-capitalized entity, currently developing and commercializing innovative treatment technologies for glaucoma. To date, the company has raised approximately $156 million through venture funding from blue chip investors. The majority of funding has been designated to research, development, and clinical trials. Glaukos has recruited and secured seasoned board members from the global health care venture community, a scientific advisory board composed of global leaders in glaucoma and comprehensive ophthalmology, and an executive team with more than 100 years of cumulative ophthalmic medical device experience.
CONCLUSION
It is estimated that 80% of glaucoma patients need mild to moderate control, and iStent and other MIGS therapies control pressure, preserve vision, and improve patient quality of life, providing exciting alternatives to medication and more invasive traditional glaucoma surgeries.
1. Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459-467.



