I am listening to a glaucoma patient tell me that he takes his prostaglandin analogue eye drop every night after brushing his teeth. His IOP is great today, but as I look at his real-time pharmacy data in our electronic health record, I see that he refilled his prescription only once in the past 6 months. When I turn the screen to show this to him, he sheepishly admits that he finds it really hard to get the eye drop into his eye, and he is embarrassed to admit that he hasn’t been very good about taking his eye drops. “On the bright side, doc,” he says, “I always take my drops before coming to see you!”
THE BIMATOPROST RING
Most ophthalmologists know that a large proportion of patients are poorly adherent to chronic glaucoma treatment and, further, that we are not very good at identifying these patients even when they are sitting right in front of us. Many of our patients would benefit from sustained-release therapy to address the many barriers to consistent adherence to long-term glaucoma treatment. One such product currently completing phase 2 trials is the Bimatoprost Ring (ForSight Vision5), a topical ocular insert designed to release up to 6 months of prostaglandin per administration. This physician-administered product has the potential to offer an alternative to patients poorly adherent to their eye drops.
The bimatoprost ring is a soft, flexible ocular insert that rests circumferentially in the fornices on top of the conjunctiva. The ring comes in multiple sizes and molds to the eye so that a comfortable, custom fit can be achieved across a wide range of patients. The ring elutes bimatoprost for up to 6 months at a time and is replaced at regular office visits by the treating provider. The Bimatoprost Ring has now been studied in nine phase 1 and 2 studies involving more than 300 patients. In the phase 1 studies, from a washout pressure of approximately 24 mm Hg, an average diurnal IOP reduction of approximately 5 mm Hg was observed for 6 months with a single administration of the ring. We recently reported the results of the first randomized, controlled phase 2 study at the American Academy of Ophthalmology meeting, and those results have been submitted for publication. Additional longer-term results will be reported later this year. Phase 3 studies of the product are anticipated to commence in 2016.
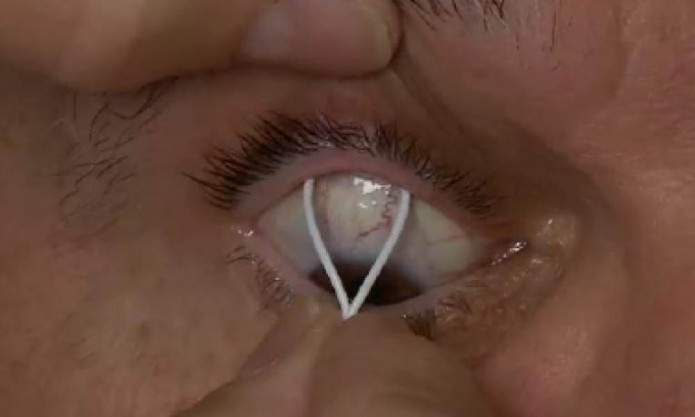
Figure 1. Placement of the upper portion of the Bimatoprost Ring.
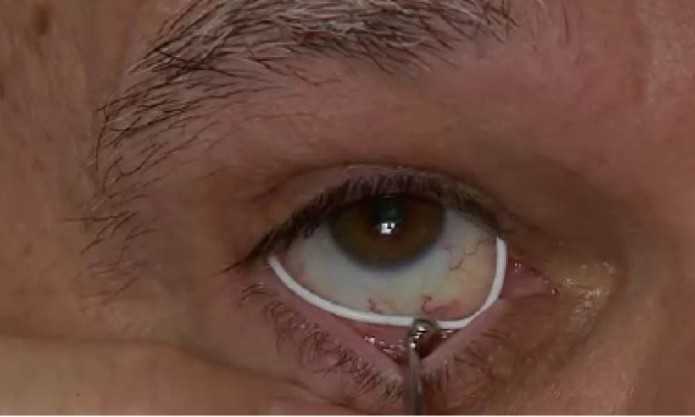
Figure 2. Placement of the lower portion of the Bimatoprost Ring.

Figure 3. Patient eye with the Bimatoprost Ring in place.
During the phase 2 clinical trials, my patients found the product to be comfortable, and most forgot it was even there. These findings were consistent with anonymous survey data from patients completing the most recent study—85% said they would recommend the product to a friend or family member who had trouble with adherence (data on file with ForSight Vision5). Importantly, among the few patients in whom the implant became partially dislodged, all noticed it immediately and were able to easily reposition it back into place, in contrast to patients whose punctal plugs fall out unnoticed.
Because the ring has a relatively high volume as compared with other extraocular drug delivery products (such as punctal plugs), the ring platform opens up the further possibility of delivering a variety of drugs or multiple drugs on a single ring. The company is working on a ring that contains both bimatoprost and timolol and also has said it plans to target dry eye and allergy indications with other formulations of the ring. In contrast to injectable platforms under development, a significant safety advantage of the topical ring platform is that in the small minority of patients in whom drug-related allergies or other significant adverse events should develop, the device can easily be removed.
SUMMARY
Data suggest that as few as 15% of patients remain highly adherent 4 years after being started on eye drops for glaucoma.1 If we can treat our lower-risk patients with a sustained-release system that lowers IOP substantially with close to 100% passive adherence, we should be able to limit the number of patients whose vision becomes functionally compromised. The bimatoprost ring offers a compelling safety profile along with IOP reduction that is expected to be clinically meaningful for lower-risk patients. As the product prepares to enter phase 3 testing, we look forward to having an even broader set of data that can be used to help bring this product to market.
1. Newman-Casey PA et al. Patterns of glaucoma medication adherence over four years of follow-up. Ophthalmology. 2015;122:2010-2021.


