Eyelashes have long been considered a focal point of the periocular area and of the facial aesthetic as a whole. To enhance lashes, cosmetic products such as mascara and eyeliner have been classically used. In the United States alone, the annual mascara market is estimated at $1.1 billion (unpublished data on file with Allergan).
Although temporary topical cosmetics remain the most commonly used method for lash enhancement, the past decade has seen an explosion of products meant to change the inherent characteristics of the lashes, making them darker, thicker, and longer. This shift began with the FDA approval of Latisse (bimatoprost ophthalmic solution 0.03%; Allergan) in 2008.
Latisse remains the only pharmaceutical approved for lash enhancement and is familiar to the majority of practicing eye care providers. However, due to the increasing popularity of some newer, less-familiar options, this article focuses on nonprescription cosmetic lash-enhancing products and their specific implications to the patient and the eye care provider.
LASH ENHANCEMENT
With the FDA approval of Latisse, the idea of products creating durable lash enhancement (thicker, longer, darker) was brought to the forefront. Classically, mascara had been used to create the appearance of enhanced lashes, but its effect is lost with removal of the product. Thus, Latisse gained immediate popularity and drove cosmetic companies to attempt to approximate the results with over-the-counter (OTC) options.
Mechanism of action. Compared with the shorter, active hair-growth stage—or anagen phase—of normal lashes, Latisse is reported to increase the duration of this phase and the percentage of total lashes grown throughout. This is what leads to the desired cosmetic effect.
Prostaglandin analogues. Several OTC products have gained popularity by claiming to achieve similar results to Latisse with the active ingredient isopropyl cloprostenate, a prostaglandin analogue. Other prostaglandin analogues used in lash products include dechloro dehydroxy difluoro ethylcloprostenolamide and methylamido dihydro noralfaprostal (Table 1).
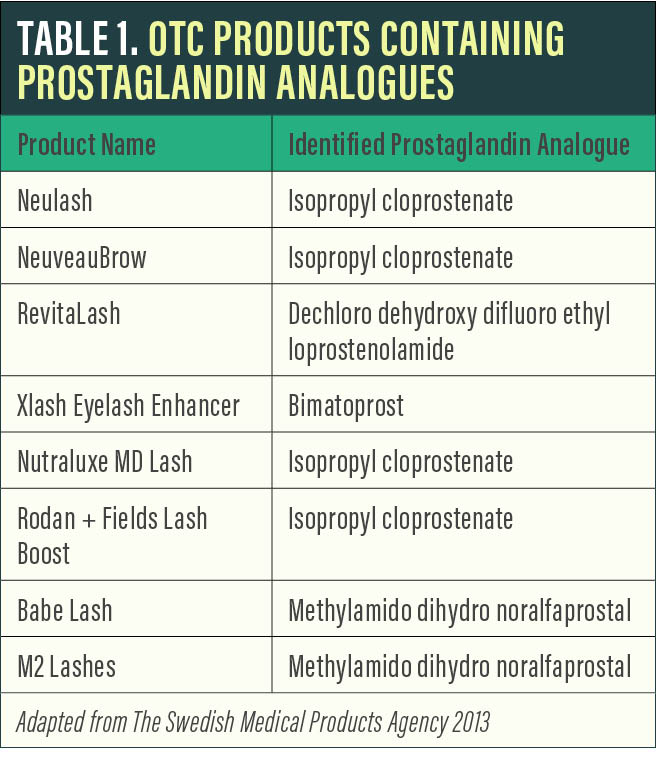
Although these products can be effective, they carry the same potential side effect profile as Latisse, including lid and ocular surface inflammation, lid and intraocular pigment changes, and orbital fat atrophy. Side effects can also be a result of the inactive ingredients present, which vary widely from product to product. Also, without FDA oversight, the ability to ensure product consistency and potential issues with individual formulations is limited.
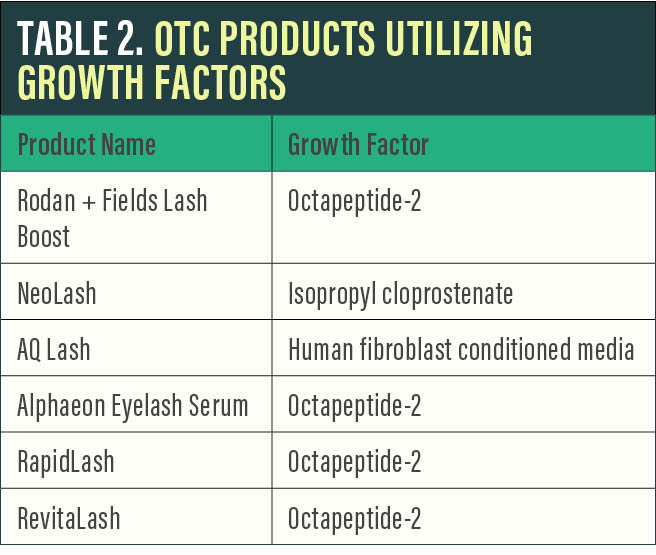
Growth factors. A growing trend in both cosmetic skin care and lash enhancement is the use of a general group of proteins referred to as growth factors. These growth factors play a role in skin cellular growth, proliferation, and differentiation. They are also believed to enhance the anagen stage, leading to longer and thicker lashes. Common growth factors in cosmetic eyelash products include octapeptide-2, keratinocyte growth factor, and human fibroblast conditioned media.
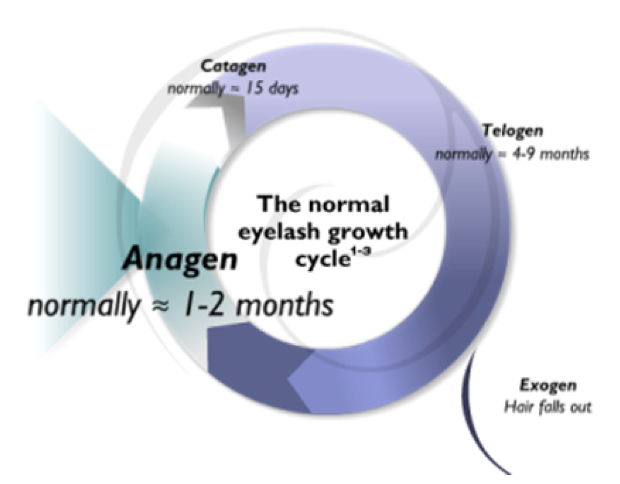
Figure 1 | Latisse increases the duration of the anagen phase of the hair-growth cycle and the percentage of eyelashes grown during this period.
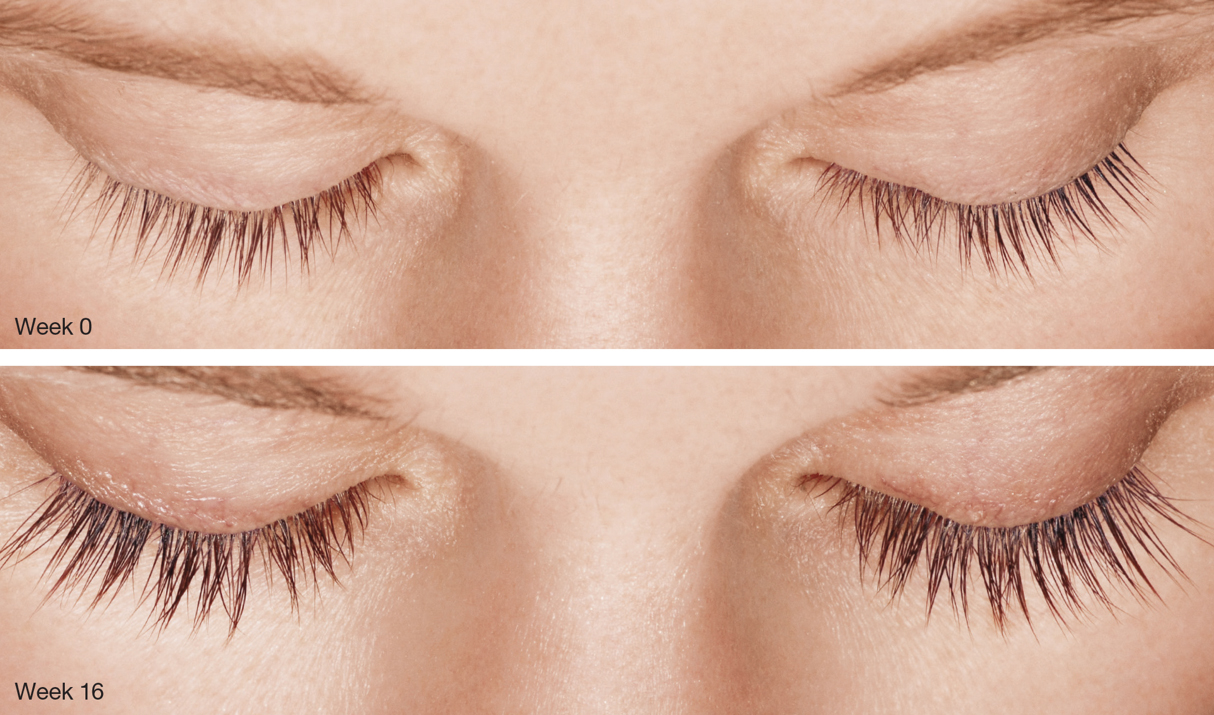
Figure 2 | Results from topical use of Latisse include longer, darker, and thicker lashes.
The role of topical growth factors in lash growth remains largely unproven. Another consideration with these products is the controversial idea that growth factors may affect the progression of skin cancer in the treated area. Although limited data exist, patients with a history of periocular skin cancer may deserve special consideration.
Peptides and other proteins. Peptides such as myristoyl pentapeptide-17 and hexapeptide-11 are meant to stimulate collagen and keratin production. Other proteins such as biotin are utilized to “support” hair growth. Lash products often include these peptides, but their utility and mechanism of action remain unclear.
Inactive ingredients. There is an incredibly wide range of inactive ingredients found in OTC lash products. Numerous products contain alcohol, which can disrupt meibomian gland function and exacerbate preexisting ocular surface disease. Many also contain ethylenediaminetetraacetic acid, which can affect the corneal epithelium. Preservatives such as benzalkonium chloride and parabens can be irritating to the ocular surface. Natural ingredients such as honeysuckle extract, pumpkin seed extract, and sweet almond extract, to name a few, have unknown effects on the complex physiology of the ocular surface.
CONCLUSION
Although Latisse remains the only FDA-approved medication for eyelash enhancement, the majority of patients using eyelash enhancement products opt for OTC products. It is vital for the eye care provider to understand the different classes of products available and the potential concerns or limitations these products may have. OTC options may be reasonable in certain scenarios; however, they provide an element of uncertainty and should be used with caution. This especially holds true for patients with preexisting blepharitis and ocular surface disease or a history of skin cancer.
1. Jones D. Enhanced eyelashes: prescription and over-the-counter options. Aesthetic Plast Surg. 2011;35(1):116-121.
2. The Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. April 15, 2013.
3. Periman LM, O’Dell LE. When beauty doesn’t blink. Ophthalmology Management. 2016;20:27,32,34,36,46.






