The detection of subclinical or forme fruste keratoconus is one of the most crucial steps in the preoperative evaluation of a refractive surgery candidate. Postoperative iatrogenic keratectasia is a rare but visually threatening complication following excimer laser corneal refractive surgery. It most commonly occurs after LASIK but has been reported following PRK and small incision lenticule extraction (SMILE).1-3 Major risk factors include surgery in eyes with undetected forme fruste keratoconus, including preoperative topographic abnormalities, low postoperative residual stromal bed thickness, younger patient age, low central corneal thickness, and high percent tissue altered (PTA).4 Postoperative keratectasia shows similar features to those seen in keratoconus and can be equally, if not more, debilitating.
A major challenge for any refractive surgeon is the detection of forme fruste keratoconus at its earliest stages. Placido topography, slit-scanning tomography, Scheimpflug tomography, and optical coherence tomography (OCT) have been utilized to identify patients at risk for postoperative ectasia. However, no system can detect all cases and predict unequivocally the risk of developing keratectasia. Combining data from newer technologies may aid in the detection of patients who are at risk of developing postoperative ectasia.
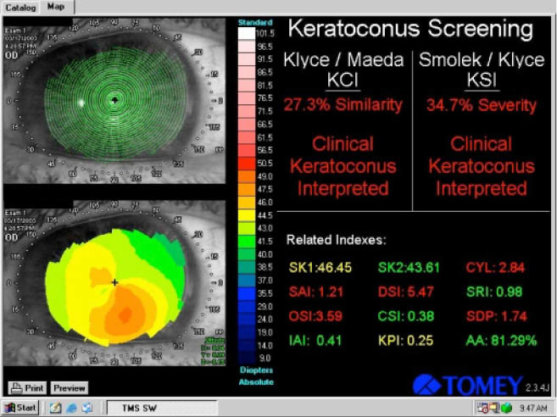
Figure 1 | Placido-based corneal topographic image display on the Tomey Corneal Topographer (Tomey). This older modality images only the anterior corneal surface. The Placido ring image (upper left) shows no significant gross abnormalities, while the anterior map (lower left) shows inferior corneal steepening. The Kylce/Maeda and the Smolek/Klyce Keratoconus screening tools available on this device can be seen (right). In the setting of preoperative refractive surgery screening, this would be concerning for frank ectactic disease and would require further evaluation.
SCORING SYSTEMS USED FOR ECTASIA SCREENING
Risk scales or scoring systems can also be useful in preoperative screening. Among the most commonly used is the Randleman Ectasia Risk Score System (ERSS),2 in which the authors propose a score that can be used to predict the risk of ectasia. This system takes into account the preoperative topography, central corneal thickness, residual stromal bed, patient age, and the planned correction. 2 Points are then assigned to each parameter on a scale of 0 to 4. The sum of these five parameters equals the patient’s relative risk of ectasia, where 0 to 2 points is low risk, 3 points represents moderate risk, and 4 or more points is high risk. The authors recommend caution with LASIK at 3 points and recommend against LASIK with 4 or more points with 96% sensitivity of and 91% specificity.2 Other scoring systems have been developed and shown similar accuracy.5
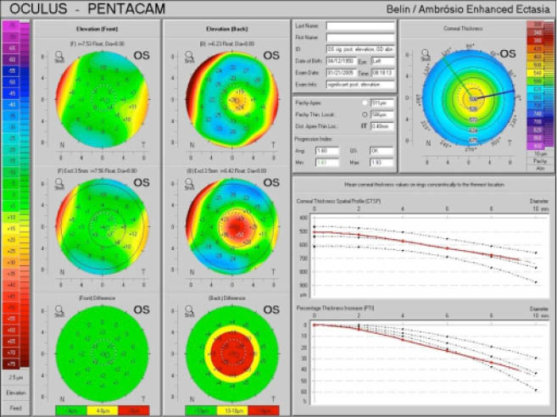
Figure 2 | The Belin-Ambrosio Enhanced Ectasia Display, showing anterior and posterior elevation maps with the standard best-fit sphere. The anterior map (upper left) shows minimal changes against the reference surface (lower left), as the anterior surface is normal. The posterior elevation (upper right) shows an early island of elevation that is exaggerated using the enhanced reference surface (lower right). Image courtesy of Irving M. Raber, MD
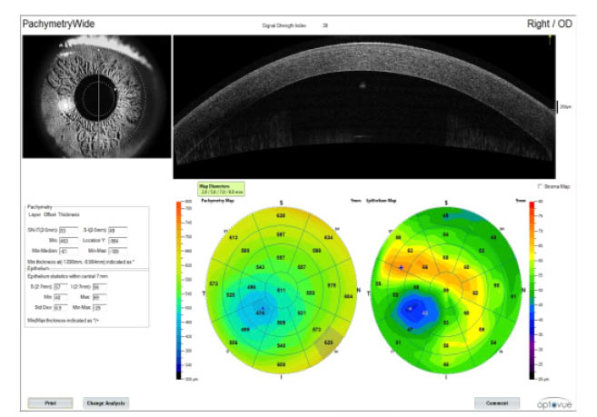
Figure 3 | Cross-sectional corneal OCT image (top right) and full pachymetric and epithelial thickness maps (bottom right), showing areas of possible ectasia.
TOMOGRAPHY-BASED SCREENING
Historically, Placido-based topography, using corneal data taken only from the anterior surface, was the mainstay of preoperative refractive screening. With the advent of tomography-based technologies, additional information on corneal shape has improved our ability to identify at-risk patients. In addition to anterior corneal data, tomography provides information about the posterior cornea and the pachymetric distribution, which can increase our ability to identify early and subtle corneal changes.5
Scheimpflug imaging is considered among the most prevalent tomographic modalities in the screening, diagnosis, staging, and follow-up of patients with keratoectasia. In addition to pachymetry and topographic imaging, Scheimpflug devices provide elevation maps of the anterior and posterior corneal surfaces.2,6,7 The main Scheimpflug devices utilized are the Pentacam (Oculus Optikgeräte) and the Galilei Dual-Scheimpflug Analyzer (Ziemer).
POSTERIOR CORNEAL ELEVATION IN SCREENING FOR KERATOECTASIA
Recent studies suggest that posterior elevation has a valuable role in the diagnosis of early ectatic disease and may be more sensitive than anterior elevation for identifying ectasia-susceptible corneas.6-9 De Sanctis et al found a high area under the curve (AUC) of 0.99 and 0.93 for posterior elevation in the diagnosis of keratoconus and subclinical keratoconus, respectively.8 Similarly, Ucakhan et al found an AUC of 0.94 when using posterior elevation to discriminate keratoconic eyes from normal eyes; however, they found a relatively lower AUC value of 0.78 in their subclinical keratoconus and normal eye comparison.7 Other studies have found the use of posterior corneal elevation to be less reliable when attempting to distinguish forme fruste keratoconus from normal eyes.10
BEST-FIT SPHERE VERSUS BEST FIT TORIC ASPHERE
Evaluation of corneal shape is an important component of the preoperative assessment of refractive surgery candidates. The most common method to assess corneal shape with a tomographer is by comparing the measured elevation data to a reference sphere or asphere.
This best-fit sphere (BFS) model on the Belin-Ambrosio Enhanced Ectasia Display (BAD), available on the Pentacam, compares the measured elevation data to a best-fit sphere (BFS) within the central 8-mm zone. It then takes the measured data and excludes a small-diameter optical zone (typically 3 mm in keratoconic corneas) centered on the thinnest portion of the cornea and recomputes the BFS reference shape. By incorporating the major ectatic region and excluding this zone from the standard BFS, a reference surface is produced that more closely mimics the more normal portions of the cornea, thus magnifying these focal areas of elevation. A back difference elevation greater than 20 μm is suggestive of ectatic disease, and a change between 10 μm and 20 μm is suspect for early ectasia.11 Using the Pentacam, Muftuoglu et al found a cutoff level of 13.2 μm to discriminate between forme fruste keratoconus and normal eyes.10
Smadja et al proposed that the use of the best-fit toric asphere (BFTA) surface is better than the BFS for discriminating between normal and keratoconic eyes and between normal and forme fruste keratoconic eyes. Using the Galilei topographer and a 6-mm zone, a cutoff of 21.5 μm was defined for the maximum elevation difference to differentiate normal corneas from those with forme fruste keratoconus.10,12
PACHYMETRIC AND EPITHELIAL THICKNESS MAPS IN SCREENING FOR KERATOECTASIA
Because keratoconus is characterized by focal thinning, a full pachymetric map generated with Scheimpflug imaging or OCT may be able to identify keratoconus cases with normal or borderline topography. Li et al used five OCT-derived pachymetric indices and determined a diagnostic cutoff for each value.13,14 They concluded that any one of the OCT pachymetric parameters that was below the cutoff was diagnostic for keratoconus and yielded an AUC of 0.99.13,14
Additionally, OCT pachymetry-based imaging has been used to evaluate early epithelial changes seen in keratoconus in an attempt to identify patients at risk of post-refractive surgery ectasia.15 Temstet et al showed that keratoconic and forme fruste keratoconic corneas had lower epithelial thickness in the thinnest corneal zone when compared with normal corneas, and an epithelial thickness threshold value of 52 μm could be used to distinguish between forme fruste keratoconic corneas and normal corneas.16 Li et al also used OCT to evaluate epithelial thickness–based variables for keratoconus detection. They found the epithelial changes seen in keratoconic eyes, compared with the normal epithelial pattern, could be detected with very high accuracy using a pattern standard deviation cutoff value of 0.057 with 100% specificity and 100% sensitivity.14,15
PERCENT TISSUE ALTERED AND THE EFFECT ON ECTASIA DEVELOPMENT
Postoperative ectasia is theorized to result from a reduction in biomechanical integrity below the threshold required to maintain corneal shape and curvature. There is an association between preoperative corneal thickness, ablation depth, and flap thickness in determining the relative amount of biomechanical change that has occurred after a LASIK procedure.17,18
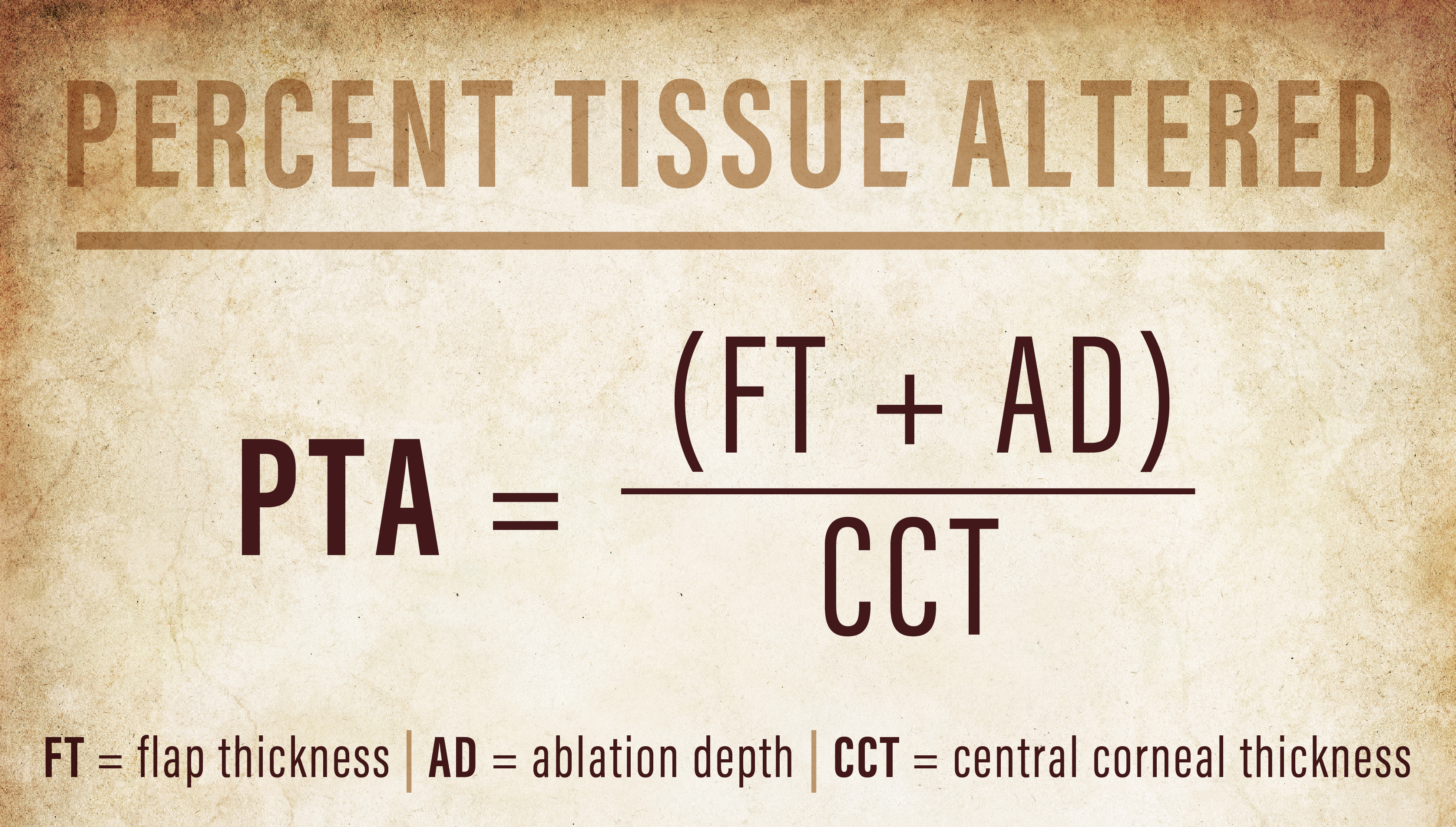
The percent tissue altered (PTA) describes this relationship, where PTA is equal to the sum of the flap thickness plus the ablation depth, divided by the central corneal thickness. This metric may more accurately represent the risk of ectasia than each measure individually. In fact, a PTA greater than 40% was the most prevalent factor for post-LASIK ectasia in one study with 97% sensitivity and 89% specificity.19 In eyes with more signs of topographic abnormalities, even less tissue alteration, or a lower PTA value, may induce ectasia.20,21
CONCLUSION
The use of new technologies in the preoperative evaluation of laser refractive surgery candidates has increased the accuracy and effectiveness of the screening process for those with significant ectasia risk. Despite rigorous screening, keratoectasia may still develop in the absence of any topographic or tomographic abnormalities. Future devices may be able to incorporate direct biomechanical testing to help identify susceptible individuals and further reduce the risk of this dreaded postoperative complication.
1. Binder PS. Ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29(12):2419-2429.
2. Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37-50.
3. Klyce SD. Chasing the suspect: keratoconus. Br J Ophthalmol. 2009;93(7):845-847.
4. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319.
5. Qin B, Chen S, Brass R, et al. Keratoconus diagnosis with optical coherence tomography-based pachymetric scoring system. J Cataract Refract Surg. 2013;39(12):1864-1871.
6. Kovacs I, Mihaltz K, Ecsedy M, Nemeth J, Nagy ZZ. The role of reference body selection in calculating posterior corneal elevation and prediction of keratoconus using rotating Scheimpflug camera. Acta Ophthalmol. 2011;89(3):e251-256.
7. Ucakhan OO, Cetinkor V, Ozkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg. 2011;37(6):1116-1124.
8. de Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008;115(9):1534-1539.
9. Chen D, Lam AK. Intrasession and intersession repeatability of the Pentacam system on posterior corneal assessment in the normal human eye. J Cataract Refract Surg. 2007;33(3):448-454.
10. Muftuoglu O, Ayar O, Ozulken K, Ozyol E, Akinci A. Posterior corneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. 2013;39(9):1348-1357.
11. Belin MW, Ambrosio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol. 2013;61(8):401-406.
12. Smadja D, Touboul D, Cohen A, et al. Detection of subclinical keratoconus using an automated decision tree classification. Am J Ophthalmol. 2013;156(2):237-246.
13. Li Y, Shekhar R, Huang D. Corneal pachymetry mapping with high-speed optical coherence tomography. Ophthalmology. 2006;113(5):792-799.
14. Li Y, Meisler DM, Tang M, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008;115(12):2159-2166.
15. Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119(12):2425-2433.
16. Temstet C, Sandali O, Bouheraoua N, et al. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract Refract Surg. 2015;41(4):812-820.
17. Buhren J, Kook D, Yoon G, Kohnen T. Detection of subclinical keratoconus by using corneal anterior and posterior surface aberrations and thickness spatial profiles. Invest Ophthalmol Vis Sci. 2010;51(7):3424-3432.
18. Ambrosio R, Jr., Caiado AL, Guerra FP, et al. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg. 2011;27(10):753-758.
19. Santhiago MR, Smadja D, Wilson SE, Krueger RR, Monteiro ML, Randleman JB. Role of percent tissue altered on ectasia after LASIK in eyes with suspicious topography. J Refract Surg. 2015;31(4):258-265.
20. Santhiago MR, Smadja D, Gomes BF, et al. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014;158:87-95.e1.
21. Santhiago MR, Wilson SE, Hallahan KM, et al. Changes in custom biomechanical variables after femtosecond laser in situ keratomileusis and photorefractive keratectomy for myopia. J Cataract Refract Surg. 2014;40(6):918-928.





