Advancements in IOL technology have assured cataract patients a better chance of achieving truly refractive results postoperatively. Because of the wide assortment of options that are available, we can offer patients a specific recommendation for a lens that will help them use their vision as desired, whether that means increased near or distance vision. With the AcrySof IQ PanOptix IOL (Alcon), we can also offer patients a lens that reliably and predictably provides vision across near, distance, and intermediate;1 the toric option also allows us to address astigmatism at the time of surgery. However, with all of the IOLs we use, the final outcome is highly dependent on the quality of biometry we are able to obtain and how accurately we can plan for the surgery.
A Solution for Better Biometry and Surgical Planning
The ARGOS Biometer with Image Guidance (Alcon) reliably answers the need for better biometry, offering fast and accurate biometry and keratometry capture,2-4 and higher acquisition rates than other market-leading biometers2,3 in an easy-to-use solution for surgical planning.5,6 The system provides angle-to-angle, cornea-to-retina, real-time OCT guidance, which allows the user to monitor patient alignment and fixation. Simultaneously, it captures a high-resolution image of the patient’s eye, enabling intraoperative eye tracking and guidance.
From a technical perspective, the ARGOS performs nine distinct measurements guided by real-time swept-source OCT in less than 1 second, including axial length (using segmented axial length as compared to a composite refractive index), corneal thickness, anterior chamber depth, lens thickness, pupil size, corneal diameter, flat and steep meridians, and astigmatism (Figure 1). Moreover, each capture includes three cycles of axial length and anterior chamber depth measurements, with the summary of measurements equating to 15 measurement cycles.5 In clinical testing, the ARGOS demonstrated faster scanning and acquisition speed than the IOLMaster 7002 and IOLMaster 5007 (both ZEISS), respectively, and greater accuracy than other biometry platforms.3,8-10
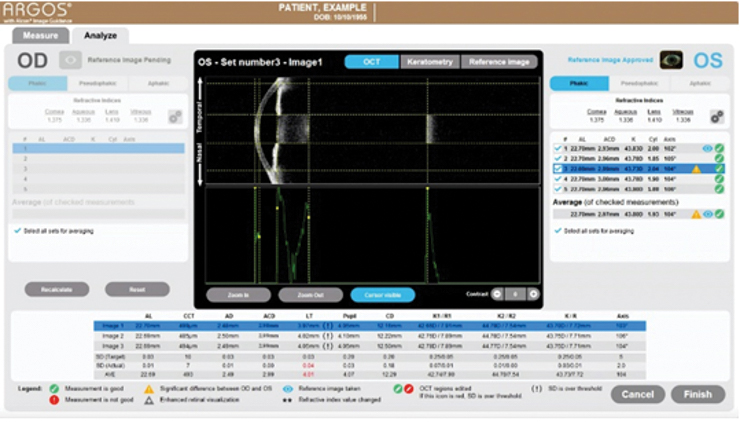
Figure 1. The ARGOS performs nine different measurements in less than 1 second.
Importantly, the ARGOS also features an enhanced retinal visualization (ERV) mode for capturing data in the setting of dense cataracts and/or opacified media.5 The 1050-nm light source permits better penetration of dense cataracts,3 thereby yielding increased sensitivity and signal detection.3,11When the signal strength is weak due to dense cataract, ERV mode can be employed to separately capture measurements in the anterior segment and through the posterior segment; these composite images are then stitched together to produce a finalized result with accurate measurements (Figure 2).5
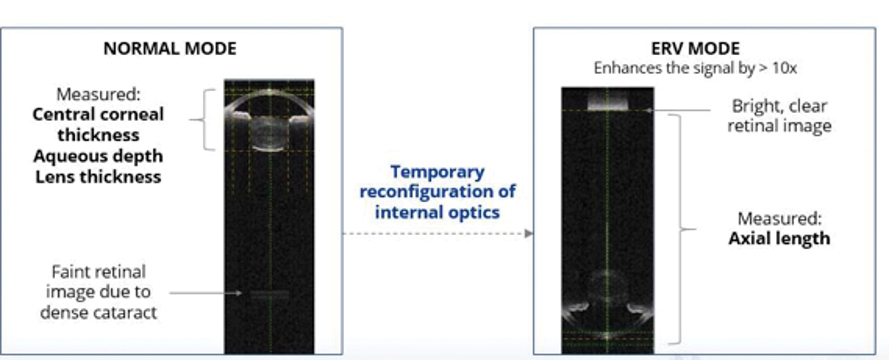
Figure 2. ARGOS biometer offers proprietary ERV mode, which helps to measure axial length through the densest of cataracts by providing 10x sensitivity at the retina, compared to Normal Mode and 100x sensitivity, compared to non-swept-source OCT Biometers.
Applications for Surgical Planning
Coupling accurate measurement data with reference imaging has great advantages for surgical planning, and in my opinion, should become the new standard for preoperative evaluation of cataract patients. Modern automated navigation applications on our smart phones provide a useful analogy for understanding the relevance of this combination: GPS data on its own is almost useless until it is matched to a reference map.
Yet, the coupling of data sources to accurate imaging is only one aspect of why ARGOS is such a powerful planning tool. For instance, the ARGOS is fully integrated with Vision Planner software (Alcon), which in turn provides great flexibility in programming user-defined preferences. Surgeons can use an extended list of IOL calculation formulae with the software, and can also preset a number of preferences, including incision location, surgically induced astigmatism, preferred IOLs, IOL constants, targeted astigmatism treatment threshold, and the targeted postoperative spherical equivalent.5
One specific example where the user-defined functions become important is in the patient with prior refractive surgery. With the latest version of Vision Planner (v1.6), the surgeon has the opportunity to manually input the posterior cornea astigmatism data generated by other devices, like Pentacam, and select for True K, total K, or the Barrett total K calculation.5
Accuracy Matters
There is only a small margin for error in helping patients achieve the postoperative vision they want. In this regard, uncorrected astigmatism can be a significant contributor to refractive surprise. Studies show that 0.75 D of astigmatism after implantation of a monofocal lens can reduce visual acuity by up to 2 lines,12 and in eyes implanted with a multifocal IOL, 0.5 D of postoperative astigmatism can reduce distance visual acuity by 1 to 2 lines.13
We recently reviewed postoperative outcomes in 38 patients implanted with a PanOptix IOL to see if using the ARGOS image-guided biometry system could help us achieve a refractive spheric target ≤0.5 D and postoperative astigmatism ≤0.5 D (Figure 3). Vision Planner using the Barrett Universal II formula was used for all patients receiving a standard PanOptix IOL. When postoperative astigmatism was calculated to be ≥0.5 D, a toric PanOptix IOL was selected for the patient, in which case the Barrett toric formula was used to calculate IOL power. The Verion Alcon Image Guidance system was used in all cases.
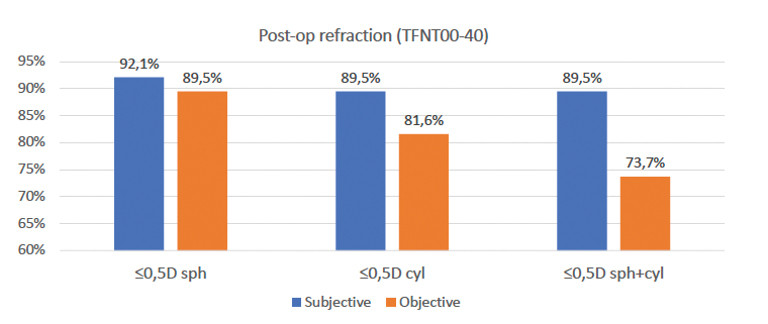
Figure 3. Postoperative refractive results among 38 patients implanted with either a standard or toric PanOptix IOL.
When we evaluated outcomes postoperatively, mean uncorrected visual acuity was 0.06 logMAR, and mean best corrected visual acuity was 0.02 logMAR. On subjective evaluation, mean absolute error for sphere and cylinder were 0.07 D and 0.07 D, respectively; on objective evaluation, these values were 0.28 D and 0.38 D, respectively. Overall, patients were satisfied with their final vision, although there is suggestion in the data that outcomes could have been refined even further: mean absolute error for cylinder among patients receiving a standard PanOptix was 0.43 D, and it was 0.33 D among patients receiving a toric PanOptix, suggesting that some patients with low astigmatism may have benefited from a toric lens. Even with this caveat, postoperative refraction results for all patients were very favorable (Figure 3).
Conclusion
The advances in biometry and surgical planning associated with the ARGOS image-guided system represent an iterative improvement in accuracy, speed of acquisition, and ease of use. These stepwise improvements are important for both the surgeon and the patient. Integrated systems help streamline clinical workflow, especially when advanced technology used in preoperative evaluation and planning functions seamlessly with the systems used intra- and postoperatively. More importantly, more accurate measurements mean a higher likelihood of making the correct lens choice for the individual patient and delivering the vision that will help them enjoy their lives postoperatively.
1. Kohnen T, Herzog M, Hemkeppler E, et al. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52-62.
2. Tamaoki A, Kojima T, Hasegawa A, et al. Clinical evaluation of a new swept-source optical coherence biometer that uses individual refractive indices to measure axial length in cataract patients. Ophthalmic Res. 2019;19:1-13.
3. Shammas HJ, Ortiz S, Shammas MC, Kim SH, Chong C. Biometry measurements using a new large-coherence-length swept-source optical coherence tomographer. J Cataract Refract Surg. 2016;42:50-61.
4. An Y, Kim H, Joo C-K. Accuracy of predicting refractive outcomes using swept-source optical coherence tomography in nuclear cataracts. J Korean Ophthalmol Soc. 2019;60(11):1043-1049
5. ARGOS® Biometer User Manual 2021.
6. VERION™ Reference Unit User Manual 2019.
7. Hussaindeen JR, Mariam EG, Arunachalam S, et al. Comparison of axial length using a new swept-source optical coherence tomography-based biometer – ARGOS with partial coherence interferometry- based biometer -IOLMaster among school children. PLoS One. 2018;13(12):e0209356.
8. Whang W, Yoo Y, Kang M, Joo C. Predictive accuracy of partial coherence interferometry and swept-source optical coherence tomography for intraocular lens power calculation. Sci Rep. 2018;8(1):13732.
9. Shammas HJ. Accuracy of IOL power formulas with true axial length versus simulated axial length measurement in 318 eyes using an OCT biometer. 2019 ASCRS ASOA Annual Meeting. May 2019.
10. Shammas HJ, Shammas MC, Jivrajka RV, Cooke DL, Potvin R. Effects on IOL power calculation and expected clinical outcomes of axial length measurements based on multiple vs single refractive indices. Clin Ophthalmol. 2020;14:1511-1519
11. Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11(18):2183-2189.
12. Moon BY, Kim SY, Cho HG, Predicting of uncorrected astigmatism from decimal visual acuity in spherical equivalent. J Opt Soc Korea. 2013;17(2):219-223.
13. Hayashi K, Hayashi H, Nakao F, Hayashi F. Influence of astigmatism on multifocal and monofocal intraocular lenses. Am J Ophthalmol. 2000;130(4):477-82.




