Nick Mamalis, MD, talked with BMC Vision about the Perfect Lens system, which can fine-tune an IOL in vivo, and the implications of adjustable IOL technology for refractive cataract surgery. If the preclinical and early clinical results with adjustable IOL technologies are positive, these developments could represent a major advance in the accuracy and customization of the cataract procedure.
BMC: How does the Perfect Lens
technology work?
Dr. Mamalis: It uses a low-grade femtosecond laser to change the refractive index of the IOL. What’s interesting is that it doesn’t work on the surface of the lens but on a layer below that’s about 50 µm thick. When laser energy hits the polymer, it induces crosslinking. The heat causes what’s called a phase separation, which produces an increase in the hydrophilicity of the material that changes the refractive index in this area (Figure 1).
Figure 1 | Eye model simulation of treatment with the Perfect Lens laser system.
Also interesting is a technique that the company calls refractive index shaping. Instead of just changing one broad area of the curvature, it’s almost like a Fresnel prism—concentric circles with this little saw-tooth arrangement. It allows more bang for the buck, if you will. A refractive index change of 0.01 can give you 3.30 D of actual change (Figure 2).
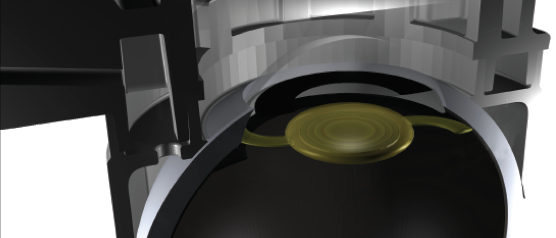
Figure 2 | Schematic illustration of refractive index shaping treatment of an IOL.
BMC: Why is that important?
Dr. Mamalis: You don’t have to do a large change of the whole surface of the lens itself. A minute change in a 50-µm area within the lens can produce a 5.00 D change or, theoretically, maybe even more.
BMC: With what sort of IOLs are investigators using this technology?
Dr. Mamalis: It can be used on hydrophobic acrylic lenses, which is by far the most common lens material used in the United States. It could also work on hydrophilic acrylic IOLs, which are more common outside the United States.
What’s exciting about this technology is that, not only can it change the spherical correction (eg, hyperopic or myopic correction), but it can also add or take off toric correction. So you could put a toric correction on an IOL to correct astigmatism, but, even more exciting, you could add or subtract multifocality. For example, if someone received a multifocal IOL and is experiencing glare and dysphotopsia and can’t tolerate it, rather than exchange the lens, you can actually put a laser pattern on there that will, in essence, erase or negate the multifocal pattern. On the other hand, if someone with a standard implant is bothered by a lack of near vision, you could put a multifocal pattern on the lens that’s already in place, giving the patient the equivalent of a multifocal lens, and see how he or she does with it. If the patient doesn’t do well, the pattern can be erased.
BMC: What is the current status of the device?
Dr. Mamalis: Extensive laboratory studies have been done on the device to see what it does to the implant itself and what it does to the optical quality. To take a step back, the precision in the change in diopters is remarkable. We’re talking about an accuracy of 0.10 D or less. Certainly, in the laboratory, it’s very precise and repeatable. We also wanted to make sure that it wasn’t disturbing the image in any way, so we did some light scattering studies on the lens itself. We found a slight increase in light scattering but nothing visually significant. When we looked at the modulation transfer function, again, there was not a significant change. So, laser treatment in the laboratory did not significantly alter the IOL itself.
We’ve already completed one rabbit study where, basically, we took a group of rabbits and put a commercially available hydrophobic acrylic lens in both eyes. We allowed the eyes to heal and the postoperative inflammation to dissipate for 2 weeks, and then we treated one lens in one eye. We did not treat the lens in the other eye. We observed them for 4 weeks after we did the treatment adjustment and looked for any signs of inflammation, after which we did histopathologic evaluations. Because the rabbit eye is a different size from a human eye, we used a custom-made adapter for the laser. Postoperative slit-lamp examinations showed no significant signs of inflammation or toxicity. We conducted postmortem histopathologic evaluations comparing the treated eyes with the control eyes and, again, found no significant inflammation or toxicity.
We are conducting a second major rabbit study. We’ve implanted the IOLs, and we’ve treated one eye and left the other eye untreated. We will observe these rabbits for 6 months. In the meantime, we are working with the FDA and the Institutional Review Board here at the University of Utah on protocols for human studies. We hope to get approval to start the clinical trials at the John A. Moran Eye Center by the end of the year. (Visit bit.ly/2H2Gr3M to watch an award-winning video on IOL power adjustments by Liliana Werner, MD, PhD, Dr. Mamalis, and colleagues.)
BMC: What need is this technology designed to meet?
Dr. Mamalis: Even though our measurements that we’re using to calculate IOL power preoperatively have improved and we’ve got better formulas and better technology to make the measurements, there are still refractive surprises and refractive outcomes that aren’t optimal, even after uncomplicated surgery (see You Can Only Be as Good as Your Tools). A history of refractive surgery in a patient can sometimes make the predictability more difficult. We do a survey here every year where we have surgeons fill out forms and send us IOLs that have been explanted, and what we’ve found is that incorrect lens power or refractive surprise is the third most common reason for explanting an IOL. Technology that modifies an IOL in vivo would be a great advance. As I alluded to previously, the technology would also benefit a significant group of patients who experience glare and dysphotopsia that would otherwise require the lens to be explanted.
You Can Only Be as Good as Your Tools
By Jack T. Holladay, MD, MSEE, FACS
Our problem today is not IOL power calculation formulas. The differences among the latest generation of those are minimal. The problem lies with the measurement errors in our tools.
The refractive outcome of cataract surgery must be within 0.50 D of the target for patients to be happy, yet less than 1% of surgeons achieve that level of accuracy 90% of the time. The average surgeon hits this refractive target approximately 75% of the time.1 Technology for measuring axial length and corneal power does not allow greater accuracy. We also cannot predict the position of the IOL with 100% certainty. The combination of axial length and corneal power measurement errors and the inability to exactly predict the position of the IOL results in an upper limit of 90% of cases within 0.50 D and a current average of 75%.2
A paradigm shift is coming in the form of technologies that will allow us to fine-tune IOLs after their implantation. RxSight has already received FDA approval for the Light Adjustable Lens, and both Perfect Lens and Clerio Vision are developing femtosecond laser technologies for the postoperative adjustment of IOLs. I anticipate that advances like these will enable cataract surgeons to achieve outcomes within 0.50 D of the refractive target in about 99% of their patients.
1. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169-178.
2. Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368-376
BMC: And thus, you’d be reducing their risk from repeat intraocular surgery.
Dr. Mamalis: Exactly. Any time we go into the eye to do a second surgery, there’s a potential risk. This procedure can be done in a laser suite and does not enter into the eye. It really allows any kind of a refractive correction that needs to be made on a lens that’s already inside the eye without a lens exchange or intraocular surgery.
BMC: What measurements would you use?
Dr. Mamalis: The IOL is already in the eye, and the eye is already healed. So the measurements are refractive.
BMC: Is there potentially less variability in the measurements, then?
Dr. Mamalis: Exactly. The eye is completely healed from surgery. You know what the spherical correction is, what the cylinder correction is, what the refraction is, and from that you can calculate what you need to do to change the power of the implant.
BMC: Have you and your fellow investigators identified any challenges with the technology in the laboratory or in the rabbit eye?
Dr. Mamalis: We haven’t had any surprises. In the lab, the studies were well done, but when you go from a laboratory setting to an actual eye, the first thing you say is, living eyes are different. There’s going to be variability there. But, when we did this rabbit study, we were aiming for a change of approximately 3.60 to 3.70 D, and when we actually did the treatment and then explanted the IOLs after the treatments and measured them to see what the change was, again, they were all within less than 0.10 D in an actually living eye. Once you got the laser coupled to the eye and lined it up properly, the treatment was very precise and actually gave us exactly what we wanted it to give us when we were looking at these rabbit eyes.
BMC: How might this technology fit with currently available options?
Dr. Mamalis: I think it gives us another way of correcting IOL power after surgery. You could still use LASIK or PRK to correct it. You could still do an IOL exchange, but to be able to do a treatment on a lens itself already in the eye, without having to work on the cornea or open the eye again and do an exchange, I think, is a great advantage. This could be done in a laser suite. It’s a very brief procedure.
BMC: How do you think the Perfect Lens technology fits in with other adjustable options?
Dr. Mamalis: RxSight’s Light Adjustable Lens (RxLAL) has been FDA approved. My colleagues and I worked with Calhoun Vision (former name of RxSight) for years as this technology was being developed. The technology is different because the specifically adjustable lens must be placed into the patient’s eye primarily. The RxLAL is a silicone lens, and a laser adjusts the power of the lens once it’s inside the eye. The advantage is, once you have the RxLAL, then you can do the proper adjustments to the power of the lens in the patient’s eye. The disadvantage is you can’t use the technology if a different lens was implanted in the eye.
BMC: What are the practical implications of the Perfect Lens technology? Will surgeons still want to implant all the various types of lenses available, or will it make more sense to implant an IOL and then tweak the result afterward? Where do you see this heading?
Dr. Mamalis: That is exactly the question that people have raised with this technology. If you have the ability to adjust the power of an IOL that’s already been put in the eye, including to add toricity or to add multifocality or to take those off, some people would advocate you just put in a standard lens, allow the eye to heal, and then adjust the power at that point. That is something that’s going to have to be worked out practically to see if that’s going to be how it works, if that’s going to be what people look at, but that certainly would be a potential for this technology.
BMC: What future directions do you envision for this technology and for the idea of adjustability as a whole?
Dr. Mamalis: The whole idea of adjustability is something that people are really interested in at the moment, and some early different types of adjustable lenses are still being looked at in animal models. Those include lenses that have two parts to them, such as the ClarVista Medical Harmoni Modular lens and the Shifamed/Atia Vision modular lens. You have a base unit inside the capsular bag. If you have to change the power, you just go in and exchange the optic, which is much less invasive than changing the entire IOL and disturbing the capsular bag. For example, in young children who have congenital cataracts, you put an implant in. As the child’s eye grows, the refractive power changes tremendously. The child is going to end up highly myopic after surgery. If we can exchange the optic for a new power, even years after surgery, that would be advantageous.




