Corneal collagen crosslinking (CXL), a procedure available commercially all over the world except in the United States, has been used therapeutically since 1998 to treat keratoconus and post-LASIK ectasia. The traditional procedure, a very straightforward application of riboflavin drops to a denuded epithelium followed by a dose of ultraviolet A (UVA) light application, once took over an hour to complete. However, research by Avedro has led to the KXL System for accelerated crosslinking, which allows a full CXL procedure to be performed in just a few minutes. The KXL System is commercially available in 60 countries outside the United States.
Now, Avedro is pushing forward to bring this technology to the United States. In November, the company announced that it received notification from the FDA that its New Drug Application (NDA) for the riboflavin ophthalmic solution/KXL System had been filed and granted priority review status. The priority review status placed the application action date at March 15, 2014; on March 18, Avedro announced that it received a complete response letter from the FDA regarding the NDA, in which the agency identified several areas of application that require additional information. In a company news release, Avedro said it will work closely with the FDA to resolve these issues as quickly as possible and is confident it can adequately answer the FDA’s questions.
The proposed indications of treatment of keratoconus and corneal ectasia following refractive surgery are both orphan indications. Orphan-drug designation is granted by the FDA Office of Orphan Products Development to promote the development of new therapies for rare diseases and disorders affecting fewer than 200,000 individuals in the United States. If approved, the riboflavin ophthalmic solution/KXL System would be the first FDA-approved crosslinking system.
This type of leadership in the field is nothing new to the Avedro team. More than 2 decades ago, as the founder and CEO of Summit Technology, current Avedro CEO David Muller, PhD, pioneered the use of excimer lasers for performing today’s laser vision correction surgery. Summit Technology became the worldwide leader in development and sales of excimer lasers for refractive correction and was acquired by Alcon for $948 million. Key members of the Summit Technology senior management team that worked with Dr. Muller to introduce excimer lasers are now working with him at Avedro to redefine refractive correction and CXL.
LASIK Xtra animation.
LASIK Xtra
The Avedro team’s research has changed the dynamic of CXL worldwide by allowing for an entirely new procedure: LASIK Xtra. LASIK Xtra is accelerated crosslinking performed intraoperatively within a standard LASIK procedure to prophylactically reduce the chances of post-LASIK ectasia and stave off regression of refractive correction. While the flap is lifted, a small amount of riboflavin is placed in the exposed corneal bed and then rinsed. The flap is repositioned as usual, and UVA light from the KXL System is applied, for a total additional treatment time of about 2 minutes.
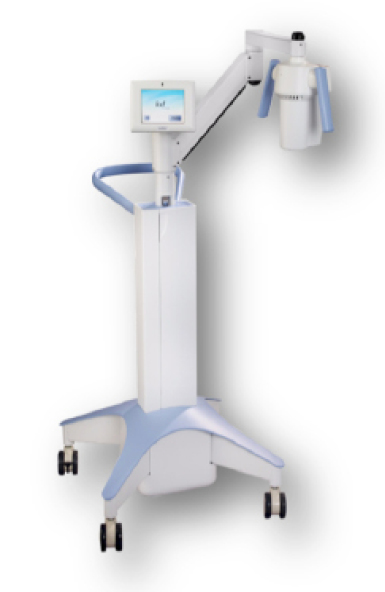
The KXL system.
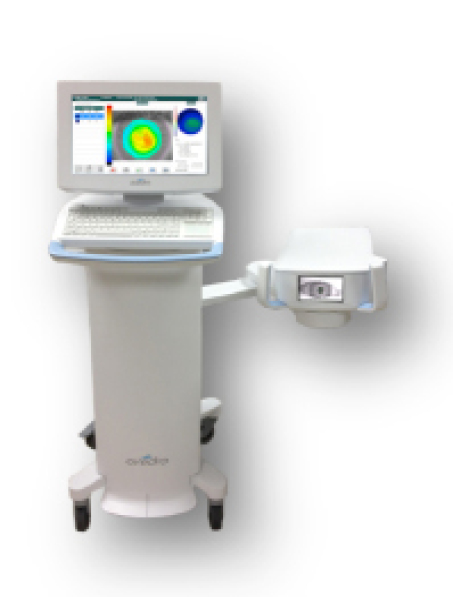
The KXL II system.
LASIK Xtra takes only a few extra minutes to perform and offers surgeons an extraordinary opportunity to provide additional assurance to their patients and to augment their practices.
More than 75,000 LASIK Xtra procedures have been performed outside the United States, where surgeons are using the treatment to promote safety and stability. In the United States, Avedro recently began clinical trials of LASIK Xtra.
A. John Kanellopoulos, MD, discusses his recent work with PiXL and the KXL II System.
PiXL
While the success of LASIK Xtra is taking off worldwide, the Avedro team is now developing new steps in refractive surgery. The KXL II System brings a new paradigm to refractive correction: the possibility for refractive change through CXL alone, via the PiXL procedure.
The refractive market is deeply underpenetrated for many reasons. PiXL aims to overcome many of the issues and provide access to an incredibly underserved population.
The KXL System is available in 60 countries and is in US clinical trials at more than 100 sites, including eight of the top 20 US eye hospitals. Avedro products are not for sale in the United States.