The Idea
Traditionally, cases of severe glaucoma were often treated with cyclophotocoagulation. This treatment ablates the ciliary body1,2 in order to reduce aqueous secretion and lower intraocular pressure (IOP). Although effective, this approach is also destructive and carries significant risks.3,4 Too much energy could cause excessively low pressures and vision problems from hypotony. Conversely, reducing the energy could result in unsatisfactory pressure lowering.
Thus, I wondered if it was possible to lower IOP without destroying the ciliary body, instead increasing the uveoscleral outflow. Around the 2010s, it was determined that topical prostaglandins increased the uveoscleral outflow through a form of digestion or breakdown, with the flow passing through the extracellular spaces within the extracellular matrix of the ciliary body. The question became whether it was possible to reproduce this effect on the matrix of the ciliary body with a laser procedure to increase normal uveoscleral output. Our team decided to use a form of cyclotherapy with which we could disrupt the cell connections that caused the pars plana pigment epithelium to be watertight without truly coagulating the site.
Our goal was to disrupt the pigmented ciliary epithelium, which was able to take up the laser energy selectively. We discovered that if you treat the pars plana, but not the pars plicata, the desired effect still occurs. We then increased the effect by treating 360° of pars plana rather than a selected amount of the population of the epithelium, disrupting the integrity of the cells rather than destroying them.
The next step was finding a device that could deliver the desired effect. At that time, MicroPulse technology (Iridex) was available and being used successfully in retina,5-7 but it was not often employed for the treatment of glaucoma. MicroPulse treatments minimize damage to tissue by delivering laser energy in short bursts, allowing tissue to cool between pulses. This is the type of delivery we needed in order to apply the laser energy without thermal buildup, causing cellular disruption without a burn effect.
Treating the pars plana—a relatively quiet site with little physiologic action—creates an outflow site. The use of nonthermal energy delivery results in pressure lowering without an increase in temperature and without much inflammation or hypotony. This course of action culminated in a treatment that was not only potent but also surprisingly associated with minimal complications. However, we needed a better delivery system.
The Process
Once the need was identified and an idea was formulated, we approached Iridex about developing a new laser probe. With the company’s partnership, we began the design process. The laser probe at the time was designed to sit firmly and securely on the eye. However, we needed a device that could slide left and right without damaging the conjunctiva surface.
Without the use of technology such as 3D printing, which was not available at the time, we drew a design that we felt would allow us to place the probe in the right location and the right distance from the limbus and designed it to slide without tearing the conjunctiva. We collaborated with Iridex’s engineers to fabricate a prototype.
The basic design was modified and refined several times. The length of the probe tip needed to be changed more than once to get it to fit the target area. The sides of the tip also needed to be refined in terms of shape to enable it to roll more smoothly. Additionally, the amount of fiber optic that protruded needed to be controlled to prevent tearing of the conjunctiva. Once we had a satisfactory prototype, it was tried in experimental situations and gradually went on to clinical trials.
A similar process could work for other surgeons who may see a need in their fields. Determine what is needed and whether or not the innovation being considered is different and valuable enough to be worthwhile. Being realistic in what is deliverable is essential. Protecting the innovation and partnering with the right company to help bring the idea to fruition is also key. The patent process that my co-collaborators and I went through differs from what is currently required. The current process for design and innovation is more systemic and more regulated, and intellectual property rights are respected in advance. A patent lawyer can assist in detailing any property rights and should be consulted.
Changing the Industry
When we began this process, our goal was to create a safer method of treatment for patients with severe glaucoma who had resisted or failed medications and who were not candidates for surgery. We desired a better, safer, and less destructive treatment than what was currently available in order to achieve a more consistent outcome with fewer side effects and complications of ablation. Destroying the ciliary body was not something we were comfortable with, although it was the only choice at the time.
Figure 1 | The MicroPulse P3 probe.
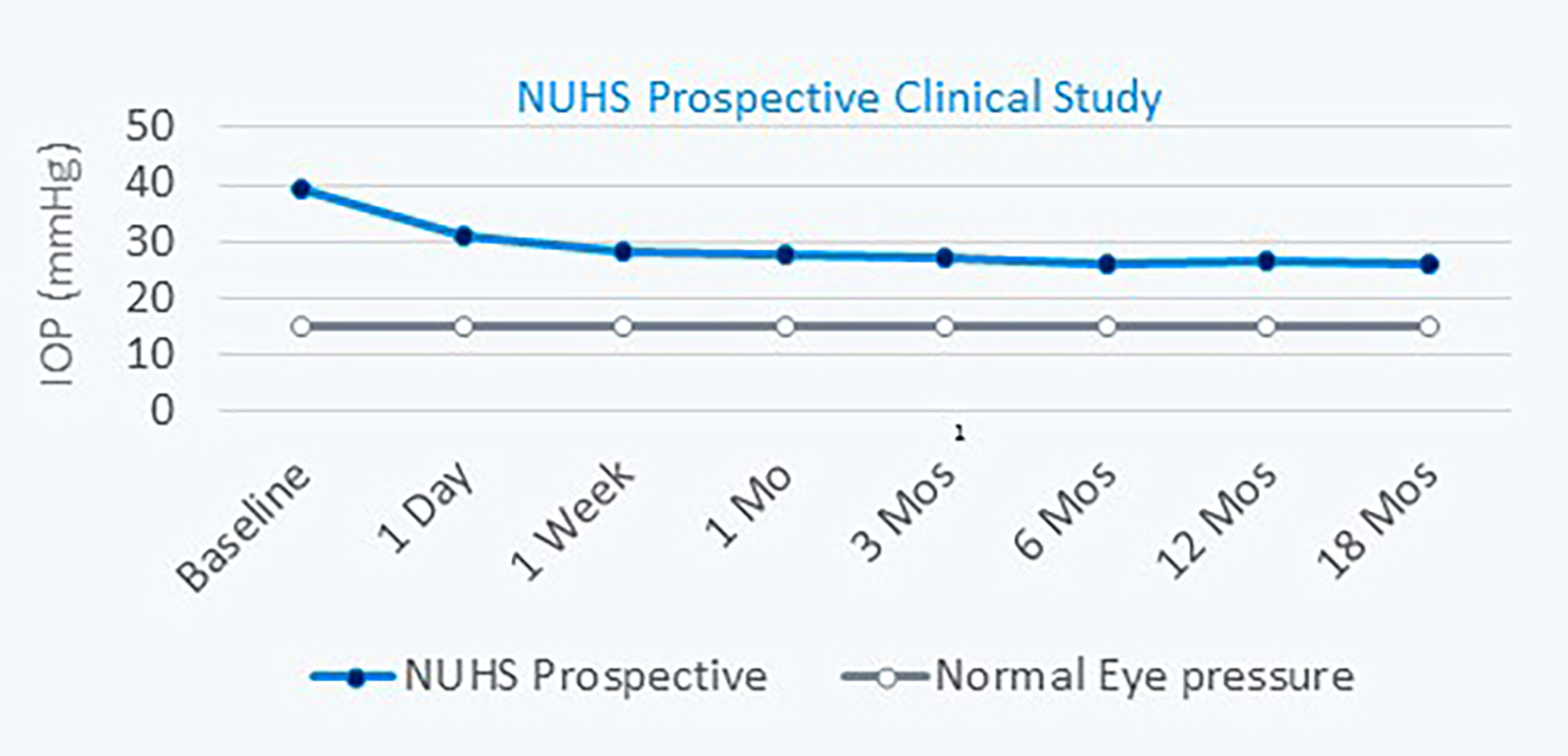
Figure 2 | Long-term study results showing a 33% IOP reduction at the 18-month follow-up.
Now, we have a treatment that is just as, if not more, potent and yet is significantly safer: the MicroPulse P3 probe (Figure 1). For patients with severe glaucoma, it is very consistent and works quite well with pressure lowering outcomes. Eyes treated with the MicroPulse P3 have little visible inflammation or retinitis and no uveitis, bleeding, or periocular swelling. Post-procedure pain is generally nonexistent. This treatment is exceptionally beneficial as a temporizing procedure for patients who have failed on medications or surgery or in eyes with poor visual potential.
We have used the MicroPulse P3 with great success to lower IOP to a safer level and reduced inflammation in order to allow for tube shunt surgery at a later date. If the treatment does not result in the necessary IOP lowering, it can be repeated. At times, patients may even be spared from the need for further surgery.
Future Outlook
The excellent safety profile of the MicroPulse P3 raises the question of whether this treatment could be utilized for milder diseases. Although this was not in our original design brief, it may indeed be advantageous for patients with earlier forms of glaucoma. We are still in the process of collecting the necessary data; however, the ability to use this treatment on a wider range of patients would be of significant benefit to all involved.
1. Feldmann RM, El-Harazi SM, LoRusso FJ, McCash CE, Lloyd WC 3rd, Warner PA. Histopathologic findings following contact transscleral semiconductor diode laser cyclophotocoagulation in a human eye. J Glaucoma. 1997;2:139-140.
2. Noecker RJ, Kelly T, Patterson E, Herrygers LA. Diode laser contact transscleral cyclophotocoagulation: getting the most from the G-probe. Ophthalmic Surg Lasers Imaging. 2004;35:124-130.
3. Caprioli J, Strang SL, Spaeth GL. Cyclocryotherapy in the treatment of advanced glaucoma. Ophthalmology. 1985;92:947-954.
4. Benson MT, Nelson ME. Cyclocryotherapy: a review of cases over a 10 year period. Br J Ophthalmol. 1990;74:103-105.
5. Sivaprasad S, Sandhu R, Tandon A et al. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow up. Clin Experiment Ophthalmol. 2007;35:640-644.
6. Parodi MB, Spasse S, Iacono P, et al. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology. 2006;113:2237-2242.
7. Desmettre TJ, Mordon SR, Buzawa DM, Mainster MA. Micropulse and continuous wave diode retinal photocoagulation: visible and subvisible lesion parameters. Br J Ophthalmol. 2006;90:709-712.




