Those who actively take care of glaucoma patients are keenly aware that these are exciting times for glaucoma management. Although still far from developing a cure for glaucoma, we are in the midst gaining greater access to promising treatment methods that offer a vast variety of options compared with what we had just a decade ago. One of those tangible down-the-pipeline options that I foresee having successful adoption in the future is the ab interno suprachoroidal shunts in the class of microinvasive glaucoma surgery (MIGS).
Excitement
What is the excitement with suprachoroidal shunts? Well, it is a whole new class of MIGS with an innovative mechanism of action that can be performed as a first-line treatment, in conjunction with other glaucoma surgical procedures, or in succession of other glaucoma surgical procedures. Remember what happened when the new class of prostaglandin analogs (PGA) hit the scene in 1996? It completely changed how we treated glaucoma. There was a reduction in the amount of glaucoma surgeries performed because adding PGAs prolonged the need for traditional surgical therapy. MIGS has been to glaucoma surgical options what latanoprost was to glaucoma drop therapy: a complete game changer. Suprachoroidal shunts now have the opportunity to add a new gear to MIGS treatment options, especially for more moderate to advanced cases.
MECHANISM OF ACTION and Products
Suprachoridal shunts aim to lower IOP by exploiting the outflow facility of the suprachoroidal space and providing a controlled and sustainable fluid egress from the anterior chamber. A secondary physiologic passageway exists, the uveoscleral pathway, in which aqueous flows into intracellular spaces in the ciliary muscle, moves through the supraciliary and suprachoroidal spaces, and finally exits the eye via absorption into the choroidal capillaries or by permeation across the sclera (Figure 1). The suprachoroidal space can be accessed through an ab interno approach using a clear corneal incision and intraoperative gonioscopy. There are two devices, one now available (CyPass; Alcon) and the other in FDA clinical trials (iStent Supra; Glaukos), that offer this connection.
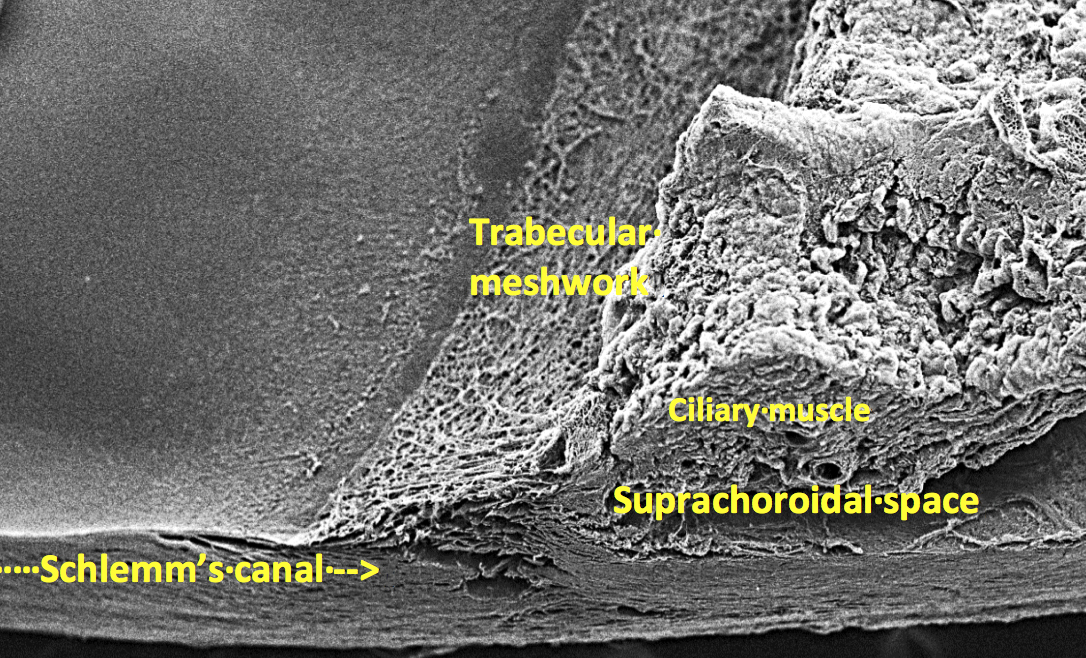
Figure 1 | Scanning electron microscopy photograph highlighting the angle structures the mechanism of action is targeting.
Courtesy of Glaukos

Figure 2 | Drawings of the iStent Supra, showing the Suprachoroidal Micro-Bypass System (A), the iStent Supra inserted into the suprachoroidal space via an ab interno approach (B), and the single-use iStent Supra loaded on the handheld inserter device.
Courtesy of Alcon
iStent Supra. The iStent Supra is a 4-mm tube made of polyethersulfone and titanium (Figure 2). It is intended to be placed in the supraciliary space, such that aqueous fluid should then be able to flow through this tube, out the small holes, and into the uveoscleral space. The device is positioned to be implanted at the time of cataract surgery in conjunction with trabecular micro bypass devices for more moderate to severe open-angle glaucoma.
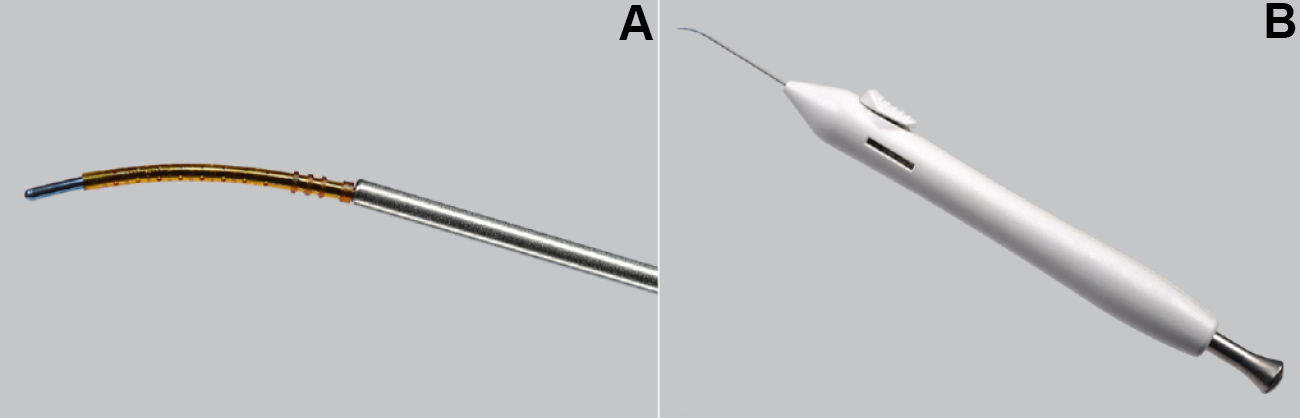
Figure 3 | The CyPass Micro-Stent loaded at the tip of the handheld inserter device (A) and the preloaded CyPass on the single-use handheld inserter device (B).
CyPass.The CyPass Micro-Stent is a small device designed to reduce IOP by enhancing suprachoroidal aqueous outflow (Figure 3). The device is a 6.35-mm-long tube with an outer diameter of 0.51 mm; it is made of a polyimide material similar to that used in IOL haptics. CyPass is now approved by the FDA to be implanted at the time of cataract surgery and positioned as a first-line treatment for mild to moderate open-angle glaucoma.
Crystal Ball Predictions
I predict that when the suprachoroidal shunts become commercially available, we will see wide adoption because they will allow for the treatment of glaucoma in the moderate to severe stages, aid in a safe and effective surgical option for IOP control, and prolong or completely eliminate the need for traditional surgery in many glaucoma patients.
1. Okeke CO. MIGS as a first-line treatment? Yes. Ophthalmology Management. February 2016: 47-49.
2. Strutton DR, Walt JG. Trends in glaucoma surgery before and after the introduction of new topical glaucoma pharmacotherapies. J Glaucoma. 2004;13:221-226.

