Under normal circumstances, most patients with glaucoma see their eye care specialist about four or five times per year. During these visits, IOP is measured with a noncontact tonometer, with a contour tonometer, or with the standard Goldmann applanation tonometer. Regardless of method, the acquisition of this clinically significant data—it is, after all, almost exclusively IOP that we treat in glaucoma—takes about 3 to 4 seconds. For a patient with four annual examinations, this represents, at most, a combined total of 12 to 16 seconds per year devoted to monitoring his or her IOP.
Glaucoma, however, is a 24-hour disease. Seeing as 1 year comprises 8,760 hours and 31,536,000 seconds, for a patient with glaucoma that leaves an undocumented IOP for more than 99.99% of the year. Further, the hours of the day when IOP spikes and fluctuations occur, typically in the night and/or early morning hours, are left in the proverbial dark. In a 2014 article overviewing the role of IOP fluctuation, Leidel et al1 concluded with the following slightly pessimistic statement: “Until a reliable method is developed that allows for constant IOP monitoring, many variables will continue to hinder us from drawing adequate conclusions regarding the significance of IOP variation.”
A NEW METHOD FOR CONTINUAL IOP MONITORING
Recently, this situation changed considerably with the introduction of a tool for continual IOP monitoring. The EyeMate-IO intraocular sensor implant (Implandata Ophthalmic Products) is a wireless IOP transducer that integrates pressure sensors, a temperature sensor, an identification encoder, an analog-to-digital encoder, and a telemetry unit into a single microelectromechanical system covered in biocompatible silicone. The EyeMate is designed for glaucoma patients undergoing cataract surgery, and it can be implanted into the sulcus like an IOL or attached to a capsular tension ring with an IOL in the capsular bag.
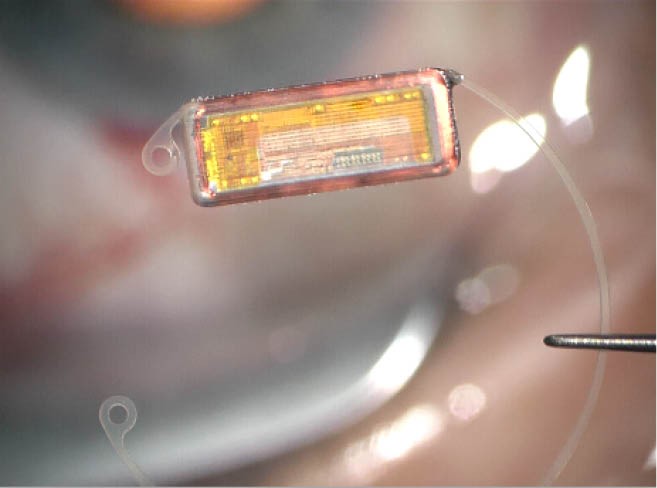
Figure 1 | The EyeMate-IO 2.0 IOP sensor from Implandata Ophthalmic Products.
The intraocular sensor is designed to stay in the patient’s eye indefinitely. It does not require a battery, but rather it derives power via electromagnetic inductive coupling to an external magnetic field generator, housed in an external reader unit. The reader unit is battery powered and, in its current design, resembles a television remote control. The same reader unit picks up the digital data relayed by the transponder unit and subsequently shows the IOP values on its light-emitting diode display. The reader and transponder units must be brought into close proximity of each other before a button is pressed on the reader to activate the electromagnetic coupling sequence—that is basically all the cooperation required from the patient.
The EyeMate sensor can conduct up to 10 IOP measurements per second. The ophthalmologist receives these measurements via telemetry, and he or she can then create a tension profile for the patient, detect dips and peaks during diurnal and nocturnal IOP fluctuations, and determine if any treatment adjustments are warranted. Using the EyeMate patient app, patients can see their IOP history and receive reminders to take their medication.
The EyeMate-IO System received the CE Mark in 2017. More than 50 of these first-generation systems have been implanted in patients at a number of clinical centers in Germany, including the University Eye Hospital in Bochum. The patient with the longest follow-up has been wearing his sensor for more than 10 years without complications. According to reports from clinical centers involved in the first trials, there appears to be a moderately increased risk of transient IOP elevation after implantation of the sensor compared with after cataract surgery alone. A moderate increase in and prolongation of postoperative anterior chamber inflammation has also been observed.
THE LATEST GENERATION OF EYEMATE TECHNOLOGY
These infrequent observations of the early EyeMate IOP sensor have been eliminated with the newest iteration of the device. The EyeMate-SC is a further miniaturized sensor that, unlike its predecessor, is implanted through a sub–3.2-mm incision into the suprachoroidal space. This enables the device to be placed in a standalone procedure, rather than only in combination with cataract surgery.
The EyeMate-SC is currently under clinical investigation in order to attain the CE Mark in 2020. We recently performed our first EyeMate-SC implantation at the University Eye Hospital, and we were impressed by the ease of the procedure, the safety, and the reliability of the data that the chip generated.
LOOKING AHEAD
Peter Szurman, MD, PhD, of the Eye Clinic Sulzbach, Germany, played a leading role in developing the EyeMate-SC and was the first surgeon to use the sensor clinically. Professor Szurman’s clinic and our University Eye Hospital in Bochum, as well as centers in Mainz, Germany, and Lausanne, Switzerland, are currently conducting a study on the safety of this fascinating new device. The independent head investigator will be Günter Krieglstein, MD, PhD, of Cologne, Germany, one of the most renowned and respected glaucoma researchers of our time.
To quote Winston Churchill, “This is not the end. It is not even the beginning of the end. But it’s the end of the beginning.” Our beginning was the age when we had insight into our patients’ IOP dynamics for just a few seconds each year—a time technology is enabling us to move beyond, bringing us one step closer to individualized glaucoma care.
1. Leidl MC, Choi CJ, Syed ZA, Melki SA. Intraocular pressure fluctuation and glaucoma progression: what do we know? Br J Ophthalmol. 2014;98:1315-1319.



