Editorially independent content supported with advertising from


Many glaucoma patients initially present with advanced disease, but in others, glaucoma progresses to an advanced stage despite careful observation by an eye care provider. Close watch of these patients is critical, because small changes may lead to a rapid and devastating collapse of functional vision—an avalanche effect. With growing disease severity, patients are at an increased risk of motor vehicle collisions and falls1,2 and suffer more from depression, social isolation, and an overall decrease in quality of life.3,4 This downward spiral has significant health and financial implications for the patient, his or her family, and society in general. It is the eye care provider's duty to remain vigilant in monitoring these patients to prevent the avalanche.
WHY IS IT DIFFICULT TO MONITOR PATIENTS WITH ADVANCED GLAUCOMA?
Despite the critical nature of advanced glaucoma, current research and diagnostic tests are mainly geared toward diagnosing and monitoring patients with less severe disease. Traditional methods of detecting progression may not be sensitive or reliable in patients with advanced glaucoma.
For example, standard automated perimetry (SAP) using the Humphrey visual field (HVF; Carl Zeiss Meditec) 24-2 testing strategy with a size III stimulus is prone to increased variability and decreased reliability in areas of low sensitivity (< 10 dB and < 15-19 dB, respectively5,6), rendering this tool less useful for advanced disease. Spectral-domain optical coherence tomography (SD-OCT) of the retinal nerve fiber layer (RNFL) is widely used to detect and monitor glaucoma, but its use is limited if the overall RNFL thickness is below the range of 50 to 60 µm, the so-called floor effect.7 Examining the cup-to-disc ratio of patients with advanced glaucoma, either at the slit lamp or with optic disc photography, is also of limited benefit because of the difficulty in detecting subtle changes in an already thin neuroretinal rim.
WHAT ARE THE BEST METHODS BY WHICH TO TRACK ADVANCED GLAUCOMA?
Objective Measures of Visual Function
SAP, typically using the 24-2 strategy, is the gold standard for monitoring the visual function of glaucoma patients. As mentioned earlier, this testing strategy is not ideal in patients with advanced disease because of large areas of low sensitivity. Identifying variability in areas of higher sensitivity (> 15 db), particularly those adjacent to areas of lower sensitivity, may be of benefit.8 Patients with an overall visual field depression to sensitivities of less than 15 dB should be switched to a size V stimulus to optimize the chances of detecting disease progression.
The 10-2 testing strategy is likely the best current objective measure of visual function for monitoring the progression of advanced glaucoma. Visual field defects approaching the central 10º of fixation are optimally observed using the 10-2 strategy because of the increased number of points (68 points) evaluated in this critical area of visual function (Figure 1). The 10-2 strategy may also identify new central defects, meeting criteria for advanced disease, that would otherwise go undetected with the 24-2 strategy.8
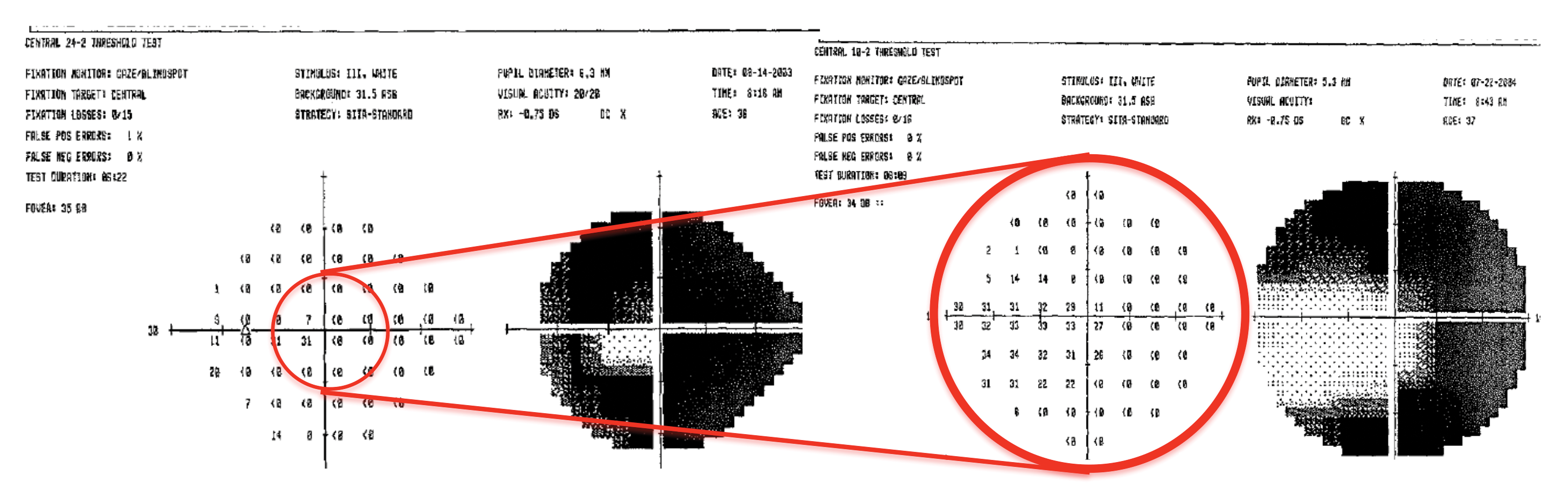
Figure 1. HVF 24-2 and 10-2 of the right eye of a patient with advanced glaucoma. The 10-2 strategy tests a greater number of locations in the central 10º, increasing its ability to detect subtle changes in this area.
Patients may not always reliably complete SAP, however, owing to difficulty fixating because of a central defect, cognitive impairment, or fatigue from the taxing nature of the test. A Goldmann visual field test (GVF), performed by an experienced—and preferably the same—technician over time, is a good option for monitoring disease progression in these patients (Figure 2). GVFs can accommodate a patient’s limitations and are particularly useful for detecting new scotomata and outlying edges of existing defects in advanced disease.
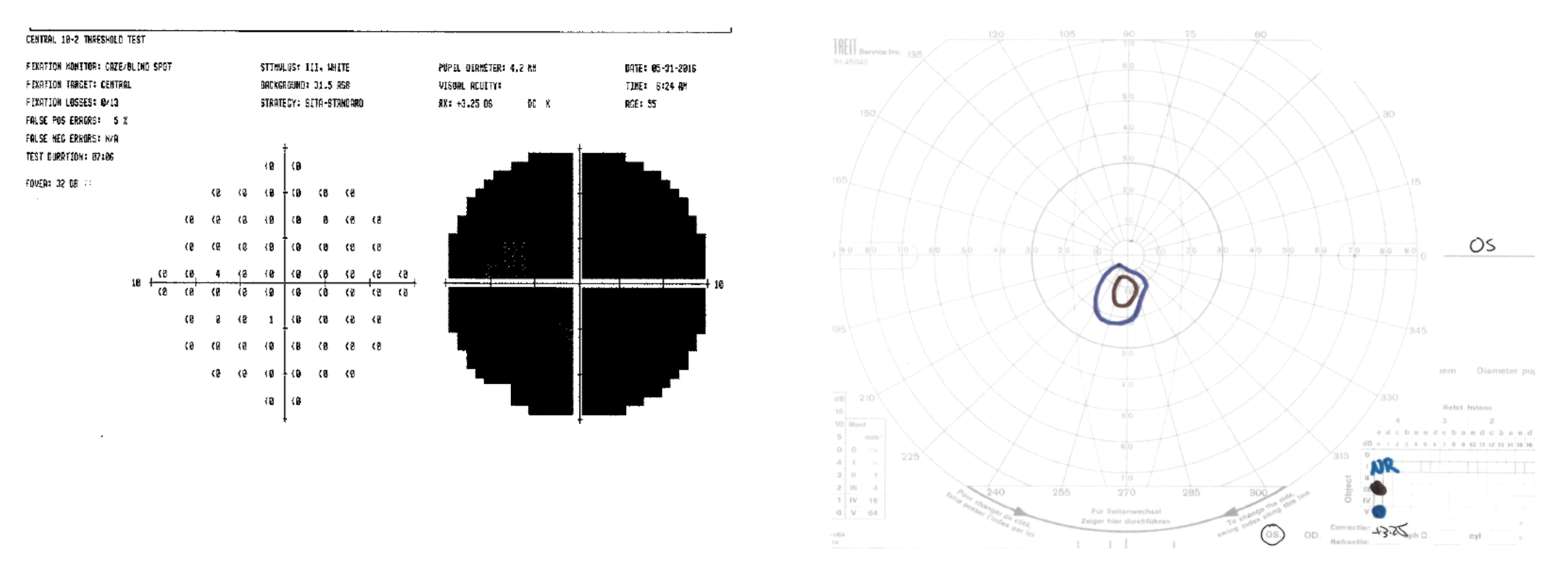
Figure 2. HVF 10-2 and GVF of the left eye of a patient with advanced glaucoma. A GVF was able to identify a small island near central vision that was not detected with a 10-2 test.
Although most clinicians use Amsler grid testing to monitor macular disease, many are unaware of its high sensitivity (92%) for detecting the progression of advanced glaucoma.9 Patients can use this simple tool at home to check for disease progression. Carefully looking for changes in other measures of vision such as distance and near visual acuity, glare testing, and contrast sensitivity (a highly underutilized measure) may also indicate a change in disease status.
Subjective Measures of Visual Function
One of the most informative methods by which to detect progression in patients with advanced glaucoma is simply inquiring about changes in their visual function. Does their vision seem to be dimmer, to vary from day to day, or to take longer to adapt to light/dark changes? Have they been struggling more lately with mobility, balance, driving, reading, or other daily activities? Such “complaints” are often dismissed if objective clinical measures are “stable,” when actually, these critical changes should alert the eye care provider to the urgent need for aggressive treatment to prevent a possible avalanche.
Structural Measures
Evaluating structural changes in the optic nerve is difficult in the advanced stages of glaucoma, because the neuroretinal rim is already thin. Serial optic disc photography is limited in its ability to detect subtle disc changes, but it can greatly aid eye care providers in identifying optic disc hemorrhages or enlargement of the beta-zone parapapillary atrophy that could be missed on examination.8
Current imaging technology such as SD-OCT of the RNFL may have some value in patients but, as mentioned earlier, reaches a floor effect.7 Recently, attention has shifted to the macular ganglion cell complex, an area with the highest density of retinal ganglion cells where SD-OCT is more likely to detect change in the late stages of glaucoma. Technological advances may produce imaging devices that are more sensitive to structural changes in patients with advanced glaucoma.
Intraocular Pressure
IOP goals for patients with advanced glaucoma should be more stringent than for those whose disease is less severe. The recommended target is the low range of normal (< 12 mm Hg) with minimal fluctuation to reduce the risk of disease progression.
HOW CAN THE AVALANCHE EFFECT BE AVOIDED?
Slight progression of advanced glaucoma may lead to a rapid and debilitating decline in patients’ visual function. Even when the disease appears to be relatively stable, frequent visits with clinical testing (every 3-4 months or more frequently), close attention to subjective changes in patients’ vision, and aggressive IOP control—likely attained with surgery—are critical. If further treatment is not an option, avenues to improve remaining visual function should be pursued. A referral to low vision services for low vision aids and to occupational therapists for an evaluation for in-home adaptation such as increased lighting10 can drastically improve patients’ quality of life.
CONCLUSION
More sensitive and reliable modalities for detecting changes in advanced glaucoma, particularly structural alterations, are needed to effectively monitor patients. Until such technology becomes available, eye care providers must carefully observe patients using current standard methods. Detecting subtle changes and providing timely and optimal care are crucial to preventing a potentially devastating avalanche of these patients.
1. McGwin G Jr, Xie A, Mays A, et al. Visual field defects and the risk of motor vehicle collisions among patients with glaucoma. Invest Ophthalmol Vis Sci. 2005;46(12):4437-4441.
2. Haymes SA, Leblanc RP, Nicolela MT, et al. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci. 2007;48(3):1149-1155.
3. McKean-Cowdin R, Wang Y, Wu J, et al. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941-948.
4. Skalicky S, Goldberg I. Depression and quality of life in patients with glaucoma: a cross-sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15. J Glaucoma. 2008;17(7):546-551.
5. Artes PH, Hutchison DM, Nicolela MT, et al. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthlamol Vis Sci. 2005;46(7):2451-2457.
6. Gardiner SK, Swanson WH, Goren D, et al. Assessment of the reliability of standard automated perimetry in regions of glaucomatous damage. Ophthalmology. 2014;121(71):1359-1369.
7. Hood DC, Kardon RH, A framework for comparing structural and functional measures of glaucomatous damage. Prog Rein Eye Res. 2007;26(6):688-710.
8. Moraes CG, Liebmann JM, Medeiros FA, Weinreb RN. Management of advanced glaucoma: characterization and monitoring. Surv Ophthalmol. 2016;61(5):597-615.
9. Su D, Greenberg A, Simonson JL, et al. Efficacy of the Amsler grid test in evaluating glaucomatous central visual field defects. Ophthalmology. 2016;123(4):737-743.
10. Bhorade AM, Perlmutter MS, Wilson B, et al. Differences in vision between clinic and home and the effect of lighting in older adults with and without glaucoma. JAMA Ophthalmol. 2013;131(12):1554-1562.