Microbial keratitis is a common sight-threatening ophthalmic emergency that requires prompt diagnosis and judicious antimicrobial treatment to mitigate ocular morbidity.1-4 Few clinical features of keratitis are specific enough to reliably diagnose the causative organism on clinical appearance alone. Bacterial keratitis causes most cases of infectious keratitis. Diagnosis, management, and outcomes can be vastly different when atypical organisms are involved. This article describes an approach to managing keratitis that fails to respond in a typical fashion to initial therapy.
AT A GLANCE
- Most cases of microbial keratitis are caused by bacteria and respond to empiric treatment with topical antibiotics.
- When keratitis fails to respond as expected, investigations and treatment can be escalated in a stepwise fashion.
- Changing antimicrobial therapy without clear guidance from investigations can cloud the clinical picture and increase the risk of complications.
THE FIRST CLUE
Most cases of microbial keratitis respond to empiric treatment with topical antibiotics.1,2 In an eye with keratitis that is responding to antibiotics, inflammation often increases for the first 1 to 2 days of treatment. As the size of the infiltrate stabilizes, its edges become more well defined—eventually organizing into a scar—and the epithelial defect heals over the previous area of keratitis.
Deviation from the expected course or regression can offer the first clue that the keratitis is atypical.
HOW TO PROCEED
When atypical keratitis is suspected, we recommend restarting the workup.
History and examination. Several features of the patient’s history may point to a particular etiology,5 including a history of herpes simplex or zoster virus, immunocompromise, organic trauma (eg, soil, contaminated water sources, agricultural activities, gardening), steroid use, ocular comorbidities, previous ocular surgery, exposure keratopathy, and lid disease.
Examination features that suggest unusual organisms include multifocal, ring, or perineural infiltrates; fluffy or fimbriated edges; and pigment within the infiltrate. Occasionally, these findings may raise suspicion of a particular organism. In some instances, however, they may simply increase the likelihood of any atypical organism and therefore prompt a more detailed investigation.
Initial culture. As soon as an atypical case is identified—whether by features of the patient’s history or examination or based on a poor response to empiric therapy—a corneal scrape and culture of the lesion should be performed, especially if they have not already been done. Culturing microbial pathogens remains the gold standard for identifying the causative organism and determining appropriate antimicrobial therapy.3,4
Broad investigation. If an organism is identified by investigations, therapy should be tailored based on laboratory-tested antimicrobial sensitivities, local antimicrobial guidelines, and expert advice. When the initial scrape fails to provide a clear answer, we recommend that any subsequent microbial testing encompass a wide range of organisms. It should include a routine Gram stain and stains specifically looking for fungal elements or parasites. It is also worth considering culturing samples on nonnutrient Escherichia coli–enriched media in addition to the standard blood agar, chocolate agar, Sabouraud dextrose agar, and thioglycollate broth.4,6
Where available, confocal corneal microscopy and polymerase chain reaction (PCR) testing (panfungal, panbacterial, herpetic, and acanthamoeba) provide a high level of specificity and reasonable sensitivity to identify the etiology of infection.7 Confocal microscopy is a noninvasive in vivo investigation that provides images comparable to those obtained with conventional histologic microscopy.6,8,9 The noninvasive imaging modality offers direct visualization of some organisms and corneal changes associated with an organism, including acanthamoeba, fungi, microsporidia, and herpetic viruses. Images can be reviewed immediately, allowing instantaneous tailoring of antimicrobial therapy. PCR assays detect the nucleic acids of organisms such as fungi, bacteria, and acanthamoeba and offer a more sensitive and rapid diagnostic modality than culture-based methods, again, permitting early targeted therapy.
Atypical keratitis may be caused by polymicrobial infection, including multiple bacteria or a combination of organisms such as herpetic virus and bacteria. We recommend broad testing for all possible causes and treatment according to clinical suspicion and laboratory results.
While awaiting the results of investigations, we recommend initiating treatment of presumed bacterial keratitis. We suggest the hourly administration of a topical second- or third-generation fluoroquinolone as monotherapy for young patients and individuals with contact lens–related keratitis. Combined fortified topical antibiotics can be used for older patients and those who have infectious keratitis associated with lid disease because of the increased prevalence of Gram-positive bacteria.2,5 With this regimen, antibiotics such as fortified cephalosporins or vancomycin often have better resistance profiles for treating Gram-positive organisms than fluoroquinolones alone.
If the first scrape fails to identify an organism and the keratitis continues to respond poorly to empiric antibiotic therapy, then a second corneal scrape should be performed after the discontinuation of antibiotics for an appropriate period. The duration of time to withhold antibiotics remains controversial,10,11 but we typically halt them 12 hours before rescraping. Should two corneal scrapes coupled with appropriate available investigations fail to find a causative organism and the keratitis is severe or progressive, then we recommend proceeding with a corneal biopsy, with one half sent for microscopy and the other half for culturing. We generally take a 2-mm partial-thickness trephination at the border of the infiltrate where the keratitis is deemed to be most active (Figure).
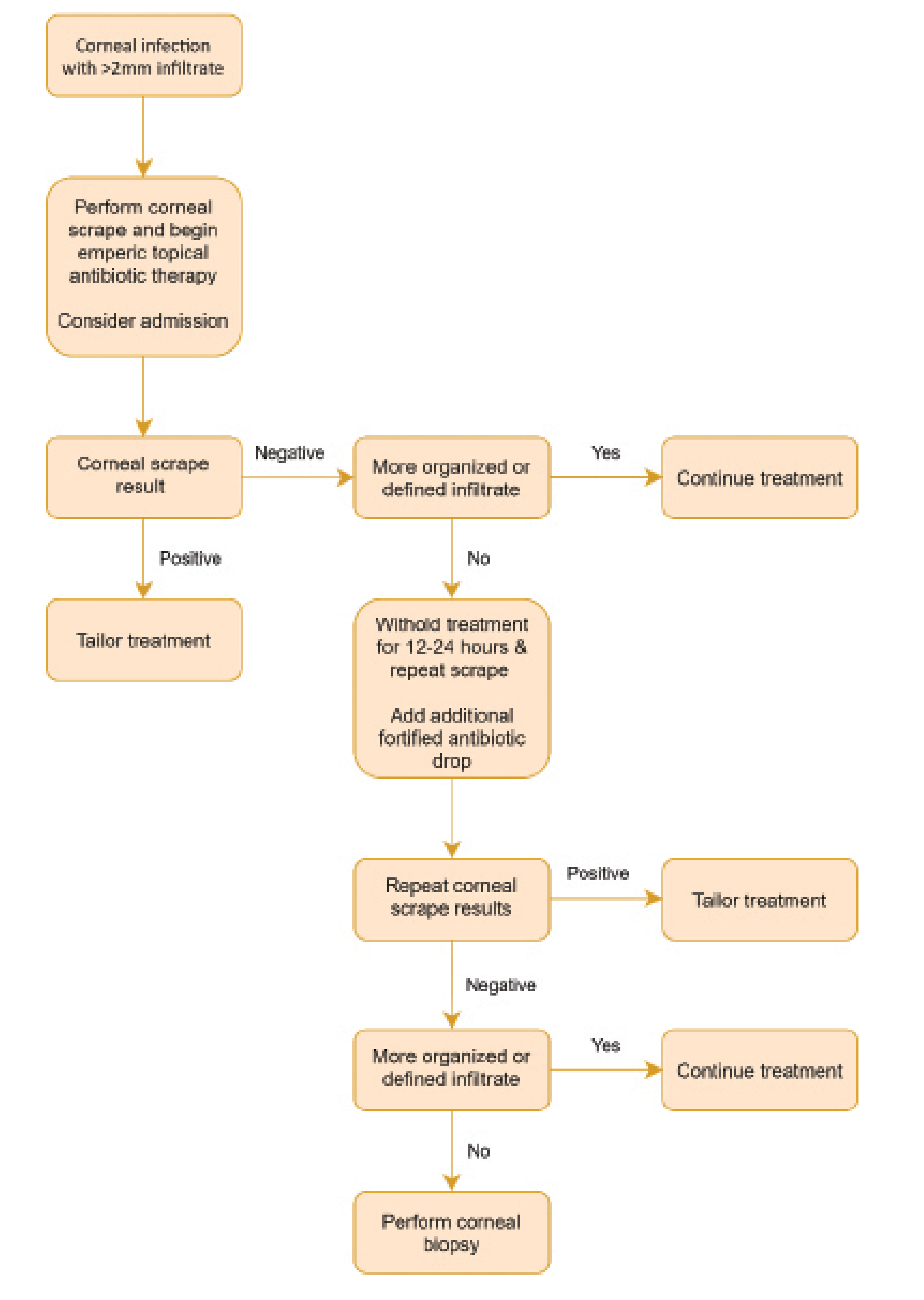
Figure. Diagnostic and treatment algorithm for microbial keratitis.
WHEN TO CHANGE THE ANTIMICROBIAL AGENT
It can be tempting to swap antimicrobial agents or add various alternative agents if the response to treatment is suboptimal. We caution against this unless the likelihood of a clinical diagnosis of a given organism is reasonably high. Treatment with the wrong agent can be harmful. It can, for example, contribute to epithelial toxicity, cloud the clinical picture, obscure the patient’s response to treatment, or increase the risk of complications such as corneal melt or perforation.
Treating presumed fungal keratitis illustrates the risks. Starting antifungal treatment before fungal disease has been confirmed can impair the ability of subsequent tests to detect fungi. This leads to a situation where the clinician is unsure what they are treating. Even when a known organism with known sensitivities is being treated, the course of fungal keratitis can be long and variable. The initiation of inappropriate therapy can result in the need for further investigation and protracted treatment.
If initial empiric antibiotic therapy fails to yield an expected response, we believe that it is reasonable to change from fluoroquinolone monotherapy to dual fortified antibiotics and vice versa. Evidence supports the efficacy of both regimens in most cases of keratitis.12,13 There is also reasonable evidence that these regimens have somewhat different sensitivities for different bacteria5 and that alterations in the antibacterial agent may treat a sensitive organism.2,6 This form of empiric swapping of an agent is reasonable for antibiotics but not for antiparasitic or antifungal agents because of the issues mentioned earlier.
CONCLUSION
When keratitis fails to respond as expected to empiric antibiotic treatment, it helps to have a plan for how to escalate investigations and treatment in a stepwise fashion. It is important to be able to recognize progressive keratitis that is failing to respond and differentiate this from a nonhealing epithelial defect. If progressive, nonresponsive keratitis develops, our preferred approach is to perform successive corneal scrapes. Should the organism remain elusive, we recommend proceeding to a corneal biopsy. A broad range of additional investigations such as PCR testing and confocal microscopy can provide valuable information. Changing antimicrobial therapy without clear guidance from investigations, however, can cloud the clinical picture and complicate the situation.
1. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22-27.
2. Cabrera-Aguas M, Khoo P, George CRR, Lahra MM, Watson SL. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin Exp Ophthalmol. 2020;48(2):183-191.
3. Bharathi MJ, Ramakrishnan R, Meenakshi R, Mittal S, Shivakumar C, Srinivasan M. Microbiological diagnosis of infective keratitis: comparative evaluation of direct microscopy and culture results. Br J Ophthalmol. 2006;90(10):1271-1276.
4. Denniston AKO, Murray PI, eds. Oxford Handbook of Ophthalmology. 3rd ed. Oxford UP; 2018:249-264.
5. Khoo P, Cabrera-Aguas MP, Nguyen V, Lahra MM, Watson SL. Microbial keratitis in Sydney, Australia: risk factors, patient outcomes, and seasonal variation. Graefes Arch Clin Exp Ophthalmol. 2020;258(8):1745-1755.
6. Khoo P, Cabrera-Aguas MP, Nguyen V, Lahra MM, Watson SL. Microbial keratitis in Sydney, Australia: risk factors, patient outcomes, and seasonal variation. Graefes Arch Clin Exp Ophthalmol. 2020;258(8):1745-1755.
7. Weisenthal RW, Rapuano CJ, Stout JT, McCannel CA. 2021-2022 Basic and Clinical Science Course, Section 08: External Disease and Cornea. American Academy of Ophthalmology; 2020.
8. Kim E, Chidambaram JD, Srinivasan M, et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol. 2008;146(5): 714-23, 723.e1.
9. Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008;2(2):435-445.
10. Garg P. Fungal, mycobacterial, and Nocardia infections and the eye: an update. Eye (Lond). 2012;26(2):245-251.
11. Lin A, Rhee MK, Akpek EK, et al; American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Bacterial keratitis preferred practice pattern. Ophthalmology. 2019;126(1):P1-P55.
12. Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK. Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J. 2009;9(2):184-195.
13. Ofloxacin monotherapy for the primary treatment of microbial keratitis: a double-masked, randomized, controlled trial with conventional dual therapy. The Ofloxacin Study Group. Ophthalmology.1997;104(11):1902-1909.
14. Daniell M, Mills R, Morlet N. Microbial keratitis: what’s the preferred initial therapy? Br J Ophthalmol. 2003;87(9):1167.




