Glaucoma management is continuing to evolve as more treatment modalities are made available to us. Surgical and device-based interventions with favorable safety profiles, coupled with improved ability to identify patients at an earlier timepoint in their disease, are changing the thinking around optimal first-line treatment. Most prominently, the idea of initiating treatment with topical medications is not necessarily a given. In addition, well-recognized issues with patients’ adherence with drop therapy is prompting earlier use of device and surgical options that mitigate cost concerns around complicated daily regimens and the potential for unwanted side effects. Although medications certainly have a role in management, the opportunities to reduce or eliminate the need for topical therapy are expanding.
Yet, while surgery and devices have unquestionably been beneficial for slowing and preventing glaucomatous progression for many patients, there is still unmet need in the treatment paradigm. To the benefit of our patients, most of the remaining questions pertain to refining the approach to patient selection and figuring out when and how to use the various treatment options. Results from clinical trials and treatment guidelines inform the approach, but decisions are typically made on a case-by-case basis.
In truth, there has always been a need to individualize the approach to treatment based on the needs of each patient: the glaucoma evaluation is predicated on building a risk profile based on the clinical examination, testing, imaging, and patient interview; determining an individualized target pressure; and then working with the patient to decide what approach will help safely achieve the desired IOP while considering impact on quality of life. The availability of new treatment options, such as the MicroShunt (Santen), has only helped to increase the likelihood of matching the right intervention to each patient. At the same time, there may still be a need for additional options that help bridge existing gaps (Figure 1).
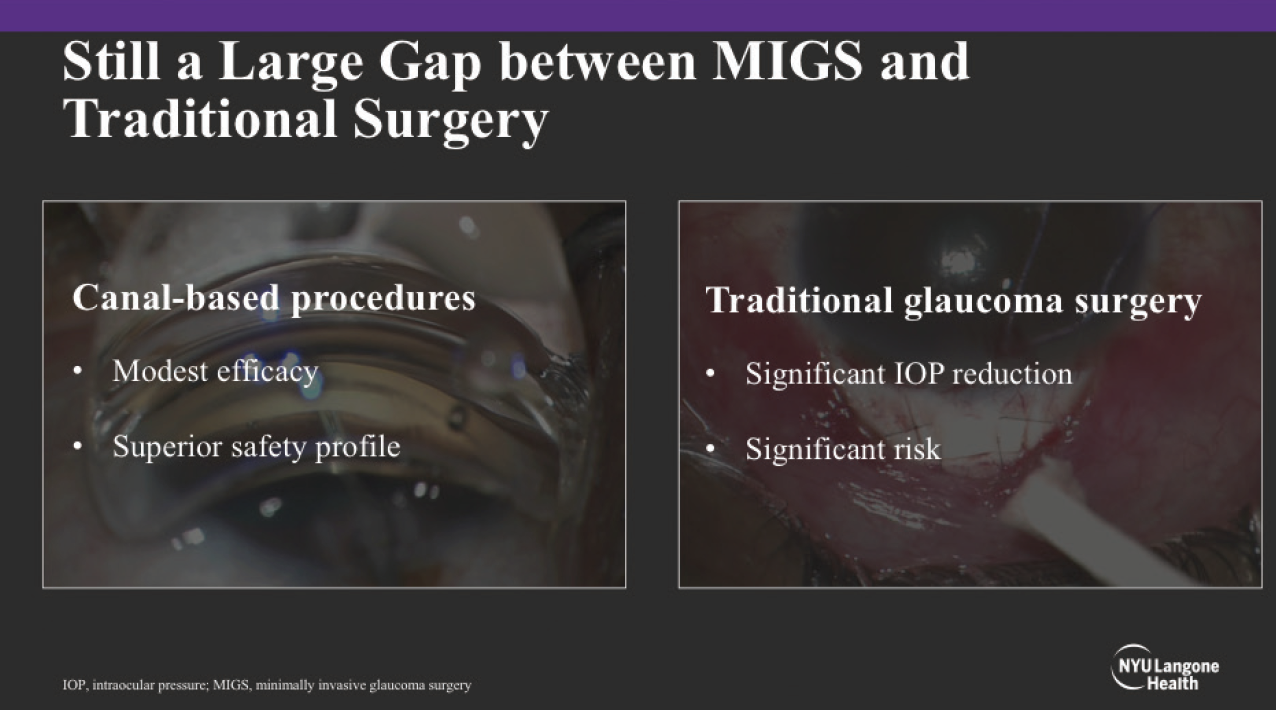
Figure 1. Subconjunctival MIGS help close the gap between canal-based procedures and traditional glaucoma surgery.
Image courtesy of Dr. Panarelli and Paul Sidoti, MD.
Rethinking Maximum Medical Therapy
The big question faced by glaucoma specialists and their patients is in figuring out where and how the various interventions fit in terms of first-, second-, and later-line treatment. Indeed, several lines of evidence suggest that individualizing decision-making to every extent possible improves the ability to achieve the ultimate goal of treatment: preventing functional blindness.
The first piece to consider is the nuance involved in determining target IOP. A full exploration of this topic is beyond the scope of this article. Nevertheless, there is considerable confusion over whether clinicians should be guided by landmark studies, whether numerical or percentile reduction in IOP is most important, and whether and how any number of clinical factors or imaging results should be used in determining target IOP. New information has also emerged on the subject of IOP fluctuation, which can be understood in several different ways, including diurnal fluctuation, intervisit variability, and short- versus long-term fluctuation. It is more than likely that all these various types of fluctuation are clinically relevant in terms of the risk for glaucomatous progression, yet without good ways to measure fluctuation, it is also uncertain how much weight the concept should be given when establishing an IOP target.
A second issue to consider overlaps with the questions around target IOP: the growing appreciation for the benefit of early intervention. Although the relationship between IOP fluctuation in its many forms and risk of progression is far from established, it is entirely reasonable that avoiding it altogether is probably better for the final outcome. More to the point, getting to the target IOP, and better yet, stabilizing the glaucoma while it is still early in its natural history, provides a better chance of slowing or stopping progression.
Even if glaucoma is recognized early and a decision is made to start an intervention, the next natural questions are what option will be most likely to achieve the goal of treatment and how sustainable will it be for the long term? The recently completed LiGHT trial helped to answer many questions about the role of selective laser trabeculoplasty (SLT), with the results suggesting that it should at least be offered in first-line settings.1 Fundamentally, the suggestion underlying the earlier introduction of SLT is to remove obstacles inherent to compliance and adherence—notably, LiGHT investigators noted that at 36 months, 74.2% of patients in the SLT group were able to maintain target IOP without drops. Yet, SLT is not universally effective, and although it is repeatable, there is often a need to continue or add medication use, which reintroduces concerns about adherence.
Historically, medication was the preferred option for early treatment phases, with decisions about advancing therapy based on the clinical circumstance. It used to be fairly common to have patients on three to four different classes of medication before moving to trabeculectomy or tube shunt surgery. With the introduction of surgical options, in particular the category of MIGS devices, there has been a rethink in what constitutes maximum medical therapy (MMT). Because of the favorable safety profile associated with MIGS, there is rationale to consider them when patients are on one to two medications—with the idea that going to surgery sooner might have the added benefit of reducing or eliminating the number of required medications. In other words, it may be the case that defining MMT numerically is insufficient, and again, an individualized approach to the question may be prudent. For instance, some patients may not want to use drops, there may be concerns about cost, side effects could be an issue, or adherence may be poor. But with the advent of MIGS, we no longer have to manage around these issues to help patients from going blind.
Considerations for Surgical Management of Glaucoma
Conceptually, surgical management of glaucoma addresses many of the issues still apparent in the treatment paradigm. As a category, surgical options largely remove the requirement for patients’ adherence. Although some of the MIGS options are only indicated for use at the time of cataract surgery, they still facilitate a greater ability to intervene more aggressively than medical therapy early in the disease course while maintaining safety. As a result, there is a better chance of getting patients to the target IOP sooner in the natural history. Altogether, the surgical management of glaucoma also obviates many of the questions around IOP fluctuation and its potential to contribute to progression.
Yet, not all surgical interventions are equal, and it is still imperative to balance safety and efficacy when deciding if a procedure will be of benefit. Traditionally, surgery benefits those patients above the target IOP on MMT, those who are progressing at the target IOP on MMT, or those who are not adherent to MMT. However, while such considerations speak to who is indicated, there is still a matter of what procedure—and there is still a gap between canal-based procedures that offer modest efficacy with superior safety and traditional glaucoma surgeries that offer more robust IOP reduction, but which are associated with significant risk.
The term “subconjunctival MIGS” has recently been coined to describe a less invasive approach to bleb formation (Figure 2). The goal is to safely and easily create a filtering bleb by shunting fluid from the anterior chamber to the subconjunctival/sub-Tenon space in a regulated manner. The length and luminal diameter of these new microshunts help restrict flow and minimize hypotony while still allowing for the formation of diffuse, posteriorly directed blebs.
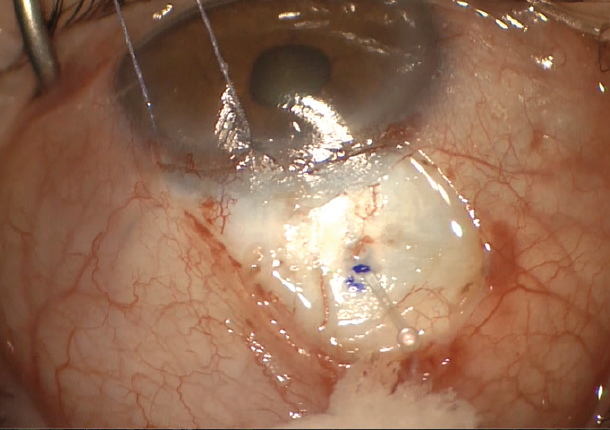
Figure 2. A novel MIGS device placed by an ab externo approach achieves bleb-based drainage from the anterior chamber to the subconjunctival space.
Image courtesy of Dr. Panarelli and Paul Sidoti, MD.
Bringing it All Together
In my practice, I weigh several factors in deciding what surgical option is appropriate for a given patient, including the type and severity of glaucoma, medication burden, health of the ocular surface, age of the patient, and how the surgery might positively or negatively impact quality of life. Of these, the latter two really speak to the art of medicine. Because we are diagnosing glaucoma at an earlier time point, and because patients are living longer and are having more active lives, we will inevitably be treating glaucoma for longer periods of time. At the same time, glaucoma itself can be devastating for patients to live with, and so there is a greater emphasis on using the appropriate tools to treat the disease when it is early and before it is progressing. To this last point, we do not want the treatment to exact any additional burden on patients’ daily living.
Within this framework, the idea of using bleb-based subconjunctival MIGS via an ab externo approach early in the natural history makes sense for a number of reasons. First, it is a reasonable option across a wide spectrum of diseases. A patient with moderate glaucoma for whom trabeculectomy is not yet indicated is a clear-cut example, but what about the young patient with mild glaucoma and a strong family history of blindness? Second, there is great potential to not just reduce but eliminate medication burden. Third, because bleb-based subconjunctival MIGS offers more potent IOP-lowering efficacy than other MIGS procedures, it has a greater chance of not just slowing but stopping progression. Fourth, the learning curve is not as arduous as some might expect. In my hands, the techniques and steps were fairly straightforward to learn and adopt.
One final compelling reason to consider earlier use of subconjunctival MIGS is the potential impact on patients’ quality of life. The procedure is associated with minimal risk to affect visual acuity or cause discomfort and minimal need for postoperative management or continued intervention. It can reduce the need for drops after surgery and has little to no impact on physical appearance. Above and beyond these procedural aspects, though, it provides an opportunity to help patients achieve target pressure earlier in their disease course, so they have a better chance of avoiding any compromise to their visual ability.
1. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al; LiGHT Trial Study Group. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-1516.

