Balancing safety and efficacy has long been an important concept in glaucoma surgery. Some of the earliest procedural interventions used in glaucoma, including goniotomy and trabeculotomy, emphasized being minimally traumatic to the eye. However, IOP-lowering efficacy was the limitation. On the other end of the spectrum are traditional bleb-based surgeries—trabeculectomy and tubes—that offer more robust IOP lowering but which are associated with greater risk, including wound leaks, suprachoroidal hemorrhage, hypotony, cystoid macular ede ma, shallow or flat anterior chamber, and aqueous misdirection during the perioperative period,1 along with risk of hypotony, blebitis, and endophthalmitis during long-term follow-up.2 The fact that these kinds of invasive surgeries still have a role is a tacit acknowledgement that some cases of glaucoma—those associated with more rapid or a higher risk of progression and thus a greater threat to visual ability—require an aggressive approach with a necessary tradeoff in terms of risk.
The most recent era in the evolution of glaucoma surgery, the introduction of MIGS, is a return to the idea of going small, thereby prioritizing safety. Because they are indicated for earlier stage glaucoma, they tend to achieve suitable IOP lowering, help stabilize the disease, and thus, lower risk of progression. Additional benefits, including reducing medication requirement, may be realized. For their intended purpose, then, MIGS elegantly deliver a balance of safety and efficacy. At the same time, the current catalogue of MIGS options may not sufficiently address the spectrum of glaucoma presentations seen in clinical practice, such as in advanced disease. There is a need for new options that offer slightly more in terms of efficacy without sacrificing the safety associated with this class.
Enhancing IOP Lowering With MIGS
MIGS devices are generally associated with a favorable safety profile; a systematic review and meta-analysis by Lavia et al found that IOP spikes were the most frequently reported complications but infection and BCVA loss due to glaucoma has not been reported.3 While there needs to be more data comparing MIGS surgery with medical therapy or other MIGS devices, the weight of available evidence provides confidence in recommending MIGS devices for a wide assortment of patient types across a spectrum of glaucoma presentations. While individual results are always variable, outcomes with MIGS devices that target conventional drainage pathways are fairly predictable, with IOP among responders resolving to the mid-teens. However, in thinking about the entire aqueous drainage process, IOP after trabecular meshwork bypass results in a pressure gradient relative to the episcleral venous pressure, which has an average pressure of around 8 mm Hg.4
Thus, while the current MIGS class is largely successful for mild to moderate glaucoma, the potential to achieve even lower IOPs without unduly affecting safety represents an unmet need. To achieve this, there are a number of potential strategies. First, one could consider combining MIGS and medications. In our lab, we have seen evidence that nitric oxide derivatives can dilate distal collector channels, thereby helping to restore physiologic aqueous drainage (unpublished data). Rho-kinase inhibitors have been shown by others to lower episcleral venous pressure.4 Thus, addition of medical therapy targeting distal outflow might synergize with the IOP-lowering efficacy of procedural approaches.
MIGS + Blebs
Another approach to achieving greater IOP lowering in a minimally invasive manner is to combine a filtering bleb to a MIGS procedure, also known as MIGS + Blebs. Combining safety and efficacy, this can create an important option across a wide range of glaucoma severity (Figure). This also explains the rationale behind the MicroShunt device (Santen). For MIGS type safety, the MicroShunt is 8.5 mm in length, with an outer diameter of 350 μm and an internal lumen of 70 μm. The lumen diameter provides just enough flow resistance to minimize hypotony. Further, resultant blebs from MIGS + Blebs are located posteriorly, which can make them less susceptible to infection and achieve better comfort and aesthetic. Together, these considerations can greatly reduce risk.
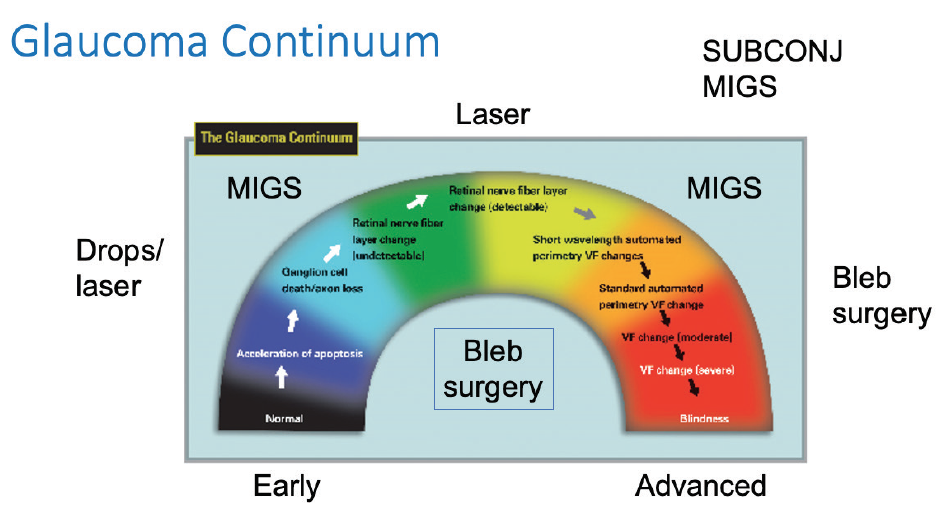
Figure. Subconjunctival MIGS is conceivably broadly applicable across a spectrum of glaucoma severity, offering more robust IOP-lowering efficacy compared to other MIGS surgeries but while maintaining the safety associated with the class.
Image modified and reproduced with permission from Weinreb RN, et al. Am J Ophthalmol. 2004.
For efficacy, bleb-based surgeries fundamentally need to accomplish three things: (1) create a connection between the anterior chamber and the subconjunctival space but without (2) resulting in scarring of the conjunctiva. Lastly, (3) the bleb must serve as a reservoir that continually drains with an exit path. By the introduction of a device, such as the MicroShunt, the first criteria is met via a minimally invasive and permanent fashion while avoiding the need to make scleral flaps.
The second criteria must also be considered, and tremendous work has already been performed with the advent of antimetabolites that reduce scarring. Antimetabolites will still be necessary in MIGS + Blebs. Future work is being done to innovate more specific agents than what we have now.
Lastly, for fluid drainage, there has been longstanding controversy about how aqueous leaves a bleb. Currently, the best evidence suggests that the aqueous that accumulates within a formed bleb is drained to the rest of the body via conjunctival lymphatic vessels. Of note, my research lab is actively involved in a number of studies to answer this question, and we anticipate publishing our data soon. For now, we can say that we have what we believe to be confirmatory evidence that lymphatic drainage plays a functional role in bleb drainage at least in most cases, and that we are doing additional research to determine whether lymphatic drainage is a necessary component of successful bleb drainage.
Taken together, by combining MIGS + Blebs the hope is to draw upon our MIGS lessons and safety profile and combine that with greater IOP reduction achieved using blebs. By further studying and better understanding subconjunctival MIGS and subconjunctival outflow, we can continue to balance safety and efficacy.
1. Jampel HD, Musch DC, Gillespie BW, et al. Collaborative Initial Glaucoma Treatment Study Group. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005;140(1):16-22.
2. Zahid S, Musch DC, Niziol LM, Lichter PR. Collaborative Initial Glaucoma Treatment Study Group. Risk of endophthalmitis and other long-term complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2013;155(4):674-680, 680.e1.
3. Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142.
4. Kazemi A, McLaren JW, Kopczynski CC, et al. The effects of netarsudil ophthalmic solution on aqueous humor dynamics in a randomized study in humans. J Ocul Pharmacol Ther. 2018;34(5):380-386.




