First introduced in the 1960s, trabeculectomy is a common type of glaucoma filtration surgery, indicated most often for patients with moderate to advanced disease, if there has been rapid progression, if prior medical management/laser/surgery has been unsuccessful, or if there is significant risk of future progression likely to yield loss of visual ability. During the surgery, a partial thickness scleral flap is created over a fistula to facilitate flow of aqueous into the subconjunctival space, thereby resulting in the creation of a filtering bleb under the conjunctiva (Figure). This mechanism results in IOP reduction that is often more profound than what is achievable with non-filtering surgeries or medical therapy. The extent of IOP reduction following trabeculectomy is variable and depends on several factors. Generally speaking, around 80% of patients achieve an IOP of 18 mm Hg or better and a 20% reduction following trabeculectomy with mitomycin C after 1 year of follow-up.1
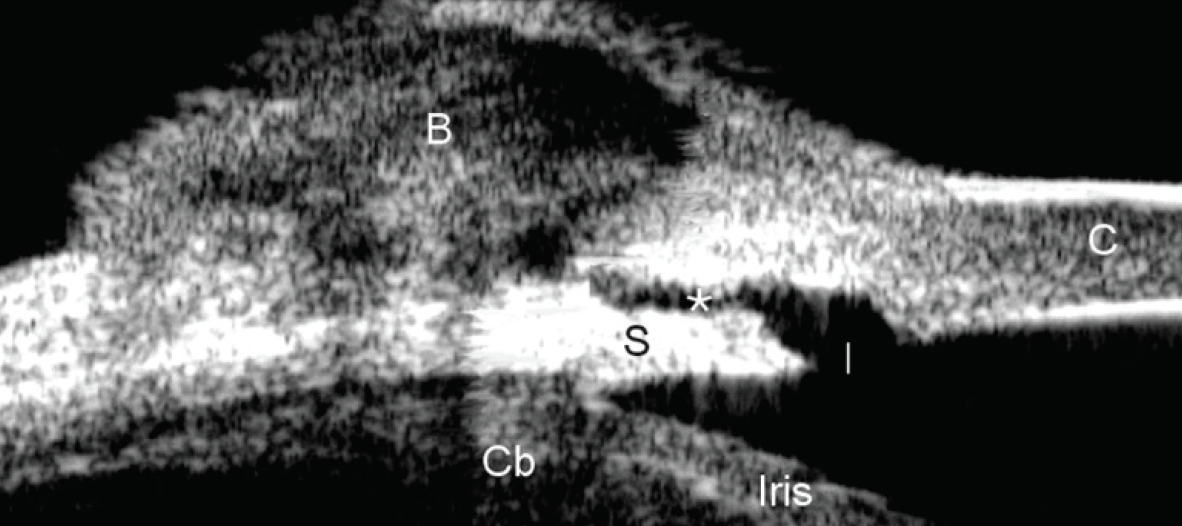
Figure. Ultrasound biomicroscopy showing a fully formed drainage bleb following trabeculectomy.
Image courtesy of Dr. Dorairaj.
However, trabeculectomy is also associated with a not insignificant rate of failure2,3 and early and late complications.4,5 Previously, in the absence of viable alternative options for patients with more severe disease, the best way surgeons could manage risks and benefits was to forestall use of trabeculectomy until absolutely necessary. Even when trabeculectomy is indicated, the surgeon has to be prepared to recognize any indication of surgical failure, and intervene quickly (ie, prescribe corticosteroids, perform a secondary procedure with filtering at the inferior limbus or use of an implant, or else revise the first filter surgery). Management of failure and complications requires a much more involved approach.
The advent of MIGS, with modest IOP-lowering efficacy but favorable safety, has helped to drive a paradigm shift in the early treatment of glaucoma. A novel microtube device placed in a MIGS procedure that affects subconjunctival drainage that is currently under investigation (MicroShunt, Santen) has the potential to help address this important unmet need in glaucoma management.
Trabeculectomy Complications
Trabeculectomy is most often considered when the potential benefit outweighs the risk, which can be understood as either the potential for the surgery to fail or for the patient to develop complications in the postoperative period. Paradoxically, even though there would seem to be a benefit to waiting as long as possible to intervene surgically (ie, only when absolutely necessary), glaucoma surgery has been identified as a risk factor for progressive visual field loss.6 Thus, it is advantageous to intervene in the earlier stages of the disease, although the options to do so are somewhat limited after the patient has progressed past mild disease. In this regard, it would be advantageous to identify patients at risk for rapid progression on their visual field, who in turn might require more aggressive management. Some risk factors have been identified, including older age, higher peak IOP, pseudoexfoliative glaucoma, and worse baseline mean deviation on visual field.6 In the latter study, previous glaucoma surgery was also identified as a risk factor, although the retrospective nature of the analysis limits a full understanding of this finding. It would be interesting to understand risk for rapid progression according to type of surgery and glaucoma severity; it is plausible that trabeculectomy and other filtering surgeries might infer greater risk for rapid progression compared to MIGS. More data are needed to improve patient selection for rapid progressors.
The list of known risk factors for trabeculectomy failure is more of a known commodity, including African descent, younger age, previous surgery on the conjunctiva, neovascular glaucoma, uveitis, and a history of topical medication use.7 In some respects, the surgery itself is a risk factor. The act of incising the conjunctiva instigates activity in the epithelial and mesenchymal cells that promote cellular proliferation, migration, and tissue remodeling.8 Likewise, tissue injury activates a number of biological processes that ultimately induce the release of a variety of proinflammatory and pro-fibrogenic mediators, such as activation of neutrophils and macrophages that release proinflammatory cytokines and chemokines (eg transforming growth factor beta [TGF-β]), and upregulated fibroblast differentiation into myofibroblasts that secrete contractile proteins and reduce tissue functionality. Angiogenesis after trabeculectomy leads to wound healing and fibrosis.
A variety of measures have been offered to reduce the potential for surgical failure and postoperative complications. Once patients are carefully selected for trabeculectomy, there is a need to cycle them off medications preoperatively that may contribute to inflammation (such as brimonidine and prostaglandins) and to start a course of oral or topical steroids. There are a number of steps the surgeon can take intraoperatively, including avoiding conjunctival buttonholes and tears, avoiding over cauterizing, and scraping episcleral tissue to prevent overgrowth. Careful monitoring in the early postoperative phase is recommended to identify whether the bleb is functional—and if it is not, options such as digital massage, removal of a suture, or use of antimetabolites (5-fluorouracil) may be employed. Over time, monitoring is continued, perhaps in conjunction with anterior chamber OCT. In cases of filtration failure, particularly in the late phases, bleb needling is a consideration. Fundamentally, what all of these measures speak to is a need for the surgeon to maintain an active role after the surgery is complete, to recognize early signs of trouble, and to act quickly to avoid unwanted outcomes.
A Different Kind of Bleb-Based Procedure
Based on our collective experience and published literature, it is safe to say that no other option is as effective as bleb-based surgeries in lowering IOP. However, not all bleb-based surgeries are equal in terms of the safety profile. In the Tube Versus Trabeculectomy (TVT) study, early complications occurred at greater frequency in the trabeculectomy group, and the rate of late complications was also higher, compared to the tube group.9 Specifically, wound leak, dysethesia, and bleb leak were each found to occur significantly more frequently in the trabeculectomy group compared to tube, whereas no early or late complications occurred at greater frequency in the tube group compared to trabeculectomy.9 Of additional note, incidence of hypotony maculopathy, and endophthalmitis/blebitis were all higher in the trabeculectomy group.9
1. Fontana H, Nouri-Mahdavi K, Lumba J, et al. Trabeculectomy with mitomycin C: outcomes and risk factors for failure in phakic open-angle glaucoma. Ophthalmology. 2006;113(6):930-936.
2. Gedde SJ, Feuer WJ, Shi W, et al. Primary Tube Versus Trabeculectomy Study Group. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 1 year of follow-up. Ophthalmology. 2018;125(5):650-663.
3. Gedde SJ, Feuer WJ, Lim KS, et al. Primary Tube Versus Trabeculectomy Study Group. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 3 years of follow-up. Ophthalmology. 2020;127(3):333-345.
4. Jampel HD, Musch DC, Gillespie BW, et al. Collaborative Initial Glaucoma Treatment Study Group. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2005;140(1):16-22.
5. Zahid S, Musch DC, Niziol LM, Lichter PR. Collaborative Initial Glaucoma Treatment Study Group. Risk of endophthalmitis and other long-term complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2013;155(4):674-680.e1.
6. Kim JH, Rabiolo A, Morales E, et al. Risk factors for fast visual field progression in glaucoma. Am J Ophthalmol. 2019;207:268-278.
7. Cabourne E, Clarke JC, Schlottmann PG, Evans JR. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst Rev. 2015;(11):CD006259.
8. Yamanaka O, Kitano-Izutani A, Tomoyose K, Reinach PS. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol. 2015;15 Suppl 1:157.
9. Gedde SJ, Herndon LW, Brandt JD, et al. Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.e1.

