
Mitchell A. Jackson, MD
Sjö
Sjögren syndrome is a serious and underdiagnosed systemic disease, as well as a significant dry eye etiology, with up to 93% of Sjögren patients suffering from dry eye.1-3 As eye care practitioners, we should be cognizant of Sjögren syndrome and testing our dry eye patients when we suspect an underlying autoimmune cause is present; current research indicates as many as one in 10 dry eye patients may have Sjögren syndrome.3 Unfortunately, many eye care professionals are neglecting this important dry eye management step, primarily due lack of awareness regarding disease prevalence. Of the 4 million estimated patients in the United States, only 1 million have been diagnosed.1-2 Additionally, the perceived difficulty of obtaining an accurate diagnosis acts as a barrier to entry. Traditional testing methods, such as salivary gland biopsy, are time consuming and associated with high false negative rates.
Fortunately, Sjö (Nicox), a recently introduced advanced diagnostic test, has greatly reduced the time and logistical challenges associated with obtaining an accurate Sjögren diagnosis in my dry eye patients. The Sjö test, which is executed via a simple finger prick—or, if a phlebotomist is present on staff, via blood draw—looks for three newly identified biomarkers that manifest early in Sjögren patients: salivary protein-1 (SP-1), carbonic anhydrase-6 (CA-6), and parotid secretory protein (PSP), in addition to the four “classic” biomarkers: ANA, RF, SS-A, and SS-B. The test takes only a few minutes to perform in office, lab results are returned within a couple of weeks, and, most important, sensitivity and specificity is very high, allowing me to detect Sjögren syndrome in dry eye patients earlier and more accurately.4
Catching Sjögren patients in the dry eye population early is critical to ensuring that they are properly cared for. As a result of lack of disease awareness and traditional testing inadequacies, the current time from manifestation of symptoms to diagnosis is 4.7 years.1 Often, these patients are bouncing around from specialist to specialist, unable to receive an accurate diagnosis. Sadly, when they do receive it, symptoms have already grown severe, forcing us as eye care professionals—as well as other medical specialists, such as rheumatologists and dentists—to proceed reactively, as it is now too late to take a proactive approach to care.
In my own practice, I utilize the Sjö test in current or new patients who present with any chronic history of dry eye. Having these new Sjögren early blood titers definitely helps guide my treatment protocol accordingly. If I receive a report indicating likely early Sjögren syndrome, I know to treat dry eye symptoms more aggressively and put the patient in touch with a rheumatologist as soon as possible to initiate comanaged care. If the report notes that Sjögren syndrome is unlikely, I know that their persistent dry eye can be attributed to another factor, and I’ll refine my hunt for the culprit. Ultimately, as the test is reimbursed by insurance and takes very little time to execute, I strive to utilize it wherever and whenever appropriate. I see confirming or ruling out this serious etiology as a best practice and would recommend adoption of the Sjö test—as well as increased education on the relationship between Sjögren syndrome and dry eye in general—to any of my peers.
Mitchell A. Jackson, MD, is a board-certified ophthalmologist specializing in cataract and refractive surgery. Dr. Jackson is the Founder/CEO of Jacksoneye in Lake Villa, Illinois, and a Clinical Assistant at the University of Chicago Hospitals. Dr. Jackson states that he is a consultant to Nicox. He may be reached at (847) 356-0700; mjlaserdoc@msn.com.
1. Sjögren’s Syndrome Foundation. 2001. Available at https://www.sjogrens.org. Accessed September 4, 2014.
2. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164:1275-1284.
3. Liew M, Zhang M, Kim E, et al. Prevalence and predictors of Sjögren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96:1498-1503.
4. Shen L, Suresh L, Lindemann M, et al. Novel autoantibodies in Sjögren’s syndrome. Clin Immunol. 2012;145:251-255.

The advanced diagnostic Sjö test has greatly reduced the time and logistical challenges associated with obtaining an accurate Sjögren diagnosis in dry eye patients.
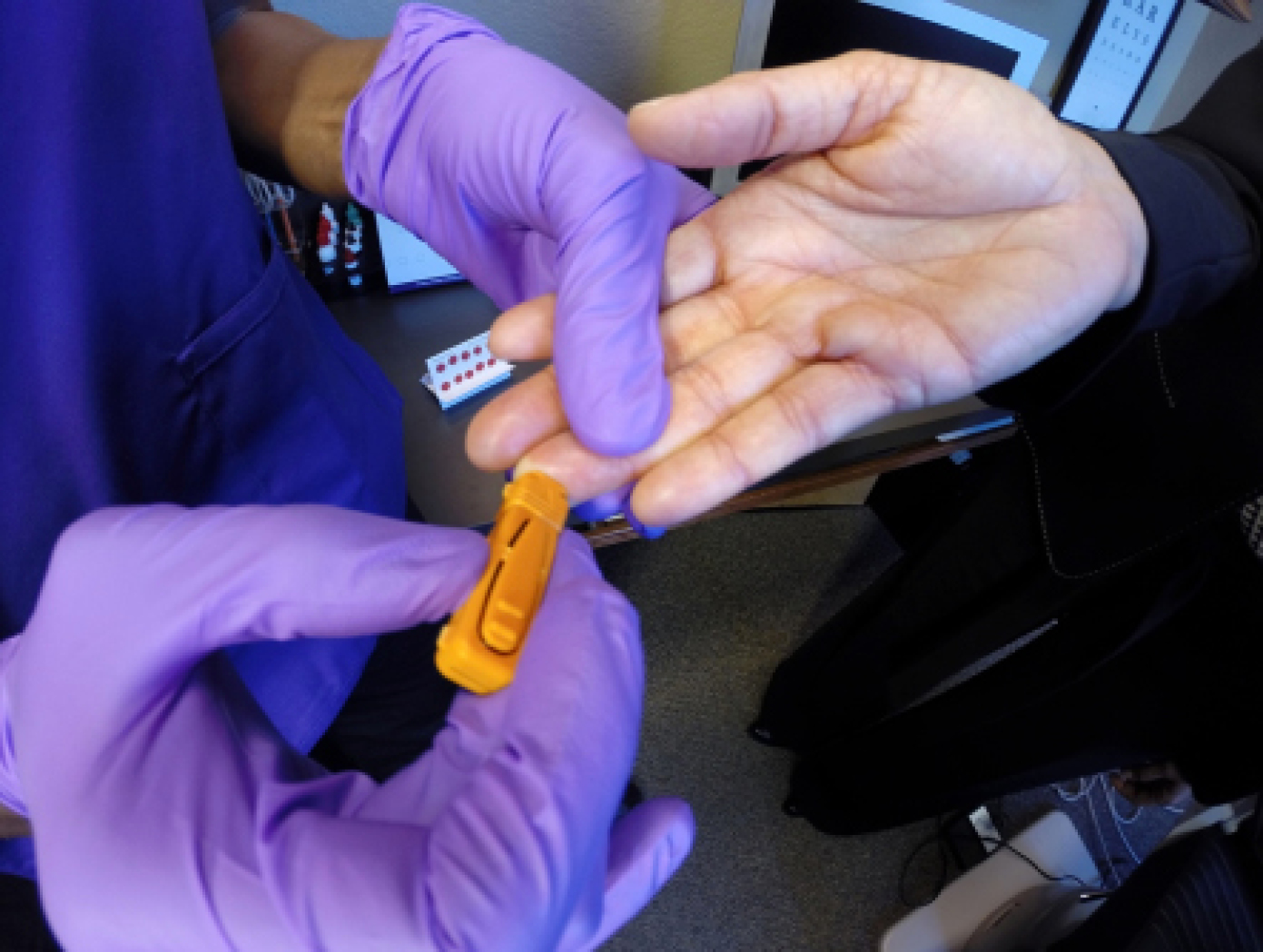
Sjö lancet finger prick procedure.

Five to ten drops of the patient’s blood placed on a Whatman card for analysis.

Kenneth A. Beckman, MD, FACS
Tear Film Osmolarity Testing
With the recent development of new diagnostic and treatment techniques for dry eye disease (DED), there has been a sudden awareness and interest in managing dry eye patients. DED is one of the most common conditions seen in the eye care professional’s office and is estimated to affect between 6 and 43 million people in the United States.1,2 For many years, DED was thought of as a nuisance disease, and many practitioners did not want to deal with these patients. The exams were time consuming, and patients were often not satisfied with their results.
As the population ages, the incidence of DED continues to rise. Patients are becoming more aware of the condition and are seeking treatment. Even as patients age, they still want to remain active, wear contact lenses, and use computers, which may exacerbate their condition. There is now a great opportunity for eye care professionals to better manage these patients and develop their own dry eye practice.
I have found that becoming a dry eye center has led to tremendous growth of my practice. In general, the patient population that presents with dry eye tends to comprise females in their 50s. This particular group of patients actually may be critical to practice growth. What I have found is that these patients tend to direct three generations of patients to the eye doctor. They are the ones who bring in their parents for cataract evaluations. They bring in their children for general eye care. They also facilitate their spouses coming in for exams. By treating their dry eyes, and making them happy, this may lead to their entire family coming to you for their eye care.
Since I started my dry eye clinic, I have found that the TearLab Osmolarity System (TearLab) has become the cornerstone of my DED evaluations and treatment. One of the main challenges in diagnosing and treating DED is that current testing is so variable and unreliable. It is very common to have a patient with a normal tear break-up time and an abnormal Schirmer test, or a normal Schirmer but corneal staining. Patient symptoms don’t seem to correlate with the exam findings. Tear film osmolarity testing now allows me to have an objective number that I can give the patient to assess his or her level of disease. While many physicians have concerns about the variability of the osmolarity results, this is actually an indicator of active disease. The unstable tear film may vary dramatically in osmolarity readings from visit to visit and between eyes. As the tear film stabilizes, not only does the osmolarity decrease, but the variability between tests and between eyes decreases as well. It is therefore necessary to collect a series of tests to determine the pattern and interpret the results.
Having access to tear film osmolarity measurements has significantly changed how I evaluate and treat dry eye patients. Whether it is for following a patient with known dry eyes or helping identify the problem in a patient with decreased vision after cataract surgery and no awareness of DED, the tear film osmolarity device has truly proven to be a game changer in my practice.
Kenneth A. Beckman, MD, FACS, is the Director of Corneal Services at the Comprehensive EyeCare of Central Ohio and a Clinical Assistant Professor of Ophthalmology at The Ohio State University in Columbus, Ohio. Dr. Beckman states that he is a consultant to TearLab. He may be reached at kenbeckman22@aol.com.
1. Beckman KA. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea. 2014;33:851-854.
2. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19-41.
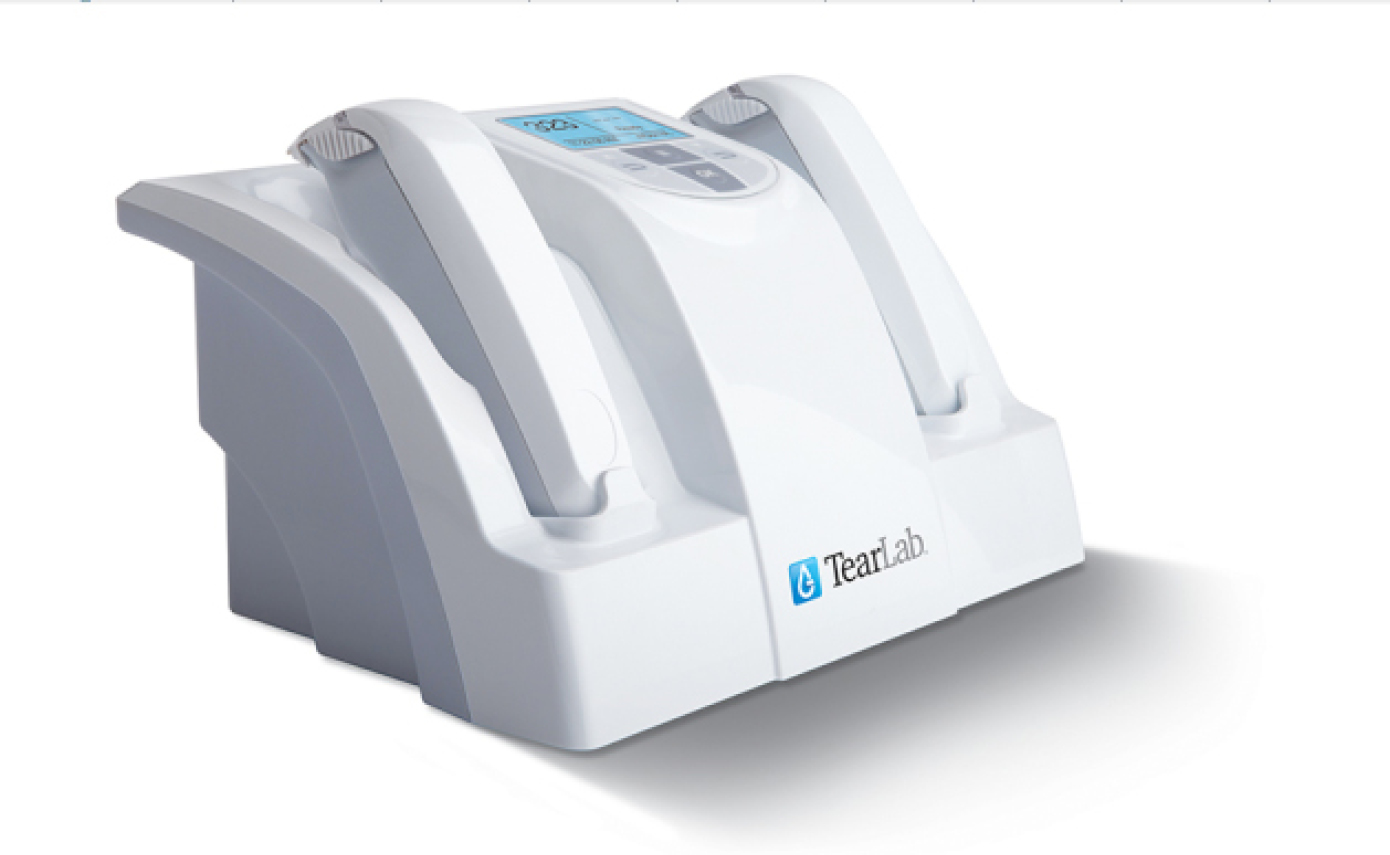
The TearLab Osmolarity System.

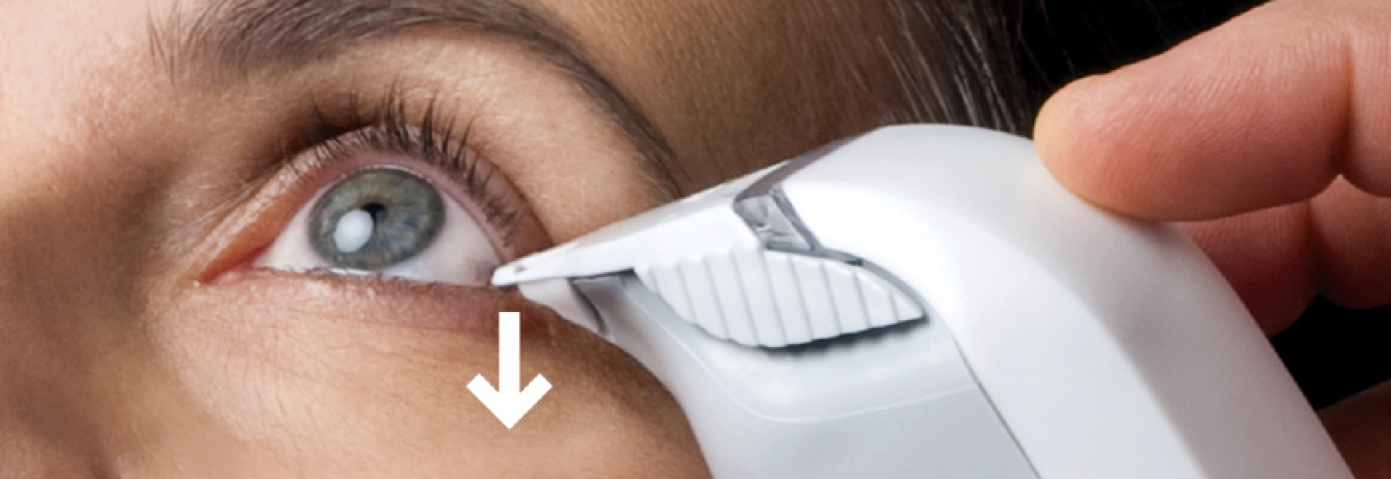
TearLab Osmolarity Test Cards and tear collection.

George O. Waring IV, MD
AcuTarget HD
The AcuTarget HD (Visiometrics/AcuFocus) is a double-pass wavefront diagnostic device that extracts light scatter from a wavefront and displays the light in terms of a point spread function. The device generates an ocular scatter index, which is essentially a functional vision score. This is indispensable for decision-making in corneal and lens-based refractive surgery and for the evaluation of what we now term dysfunctional lens syndrome.1 This condition, which has been overlooked and inadequately characterized for years, describes progressive worsening of presbyopia and the decline in quality of vision due to lenticular opacification and higher-order aberrations associated with the aging of the crystalline lens. With the AcuTarget HD, we can objectively measure a patient’s quality of vision, which aids in both treatment planning and patient education. The AcuTarget HD can also perform tear film assessment by measuring light scatter from tear film instability and demonstrating how dry eye affects the patient’s quality of vision. Other capabilities include the measurement of pseudoaccommodation to visually demonstrate depth of focus and centration guidance for Kamra inlay (AcuFocus) implantation. The AcuTarget HD is also useful for examining patients with complaints of image quality or polyopia.
The AcuTarget HD enhances our ability to identify factors that could impact postoperative outcomes, improves our understanding of patients’ quality of vision, and enables us to refine our treatment strategies for optimal results—a true game changer in refractive surgery.
George O. Waring IV, MD, is the Director of Refractive Surgery and an Assistant Professor of Ophthalmology at the Storm Eye Institute, Medical University of South Carolina. Dr. Waring is also the Medical Director of the Magill Vision Center in Mt. Pleasant, South Carolina, and the Chief Medical Editor of MillennialEYE. He is a consultant to AcuFocus and a medical director for Visiometrics. Dr. Waring may be reached at waringg@musc.edu.
1. Waring GO IV. Diagnosis and treatment of dysfunctional lens syndrome. Cataract & Refractive Surgery Today. 2013;13(3):36-38.

The AcuTarget HD.
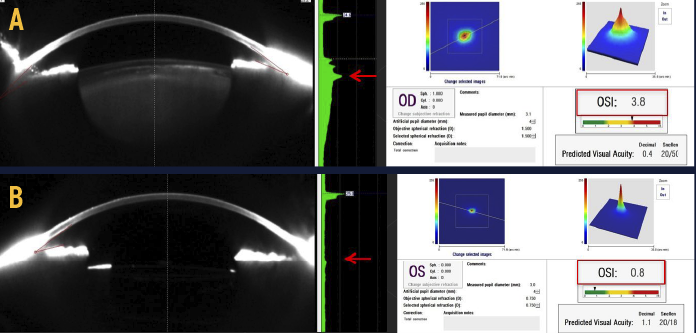
The AcuTarget HD can be used to objectively measure a patient’s quality of vision and aid in the diagnosis of dysfunctional lens syndrome. Shown here is a patient’s untreated right eye (A) compared with her left eye following dysfunctional lens replacement (B). Notice the differences in point spread function, OSI, and densitometry.

Sumitra S. Khandelwal, MD
Anterior-Segment OCT
As surgeons, if we consider where we are with new techniques and technologies, it is fairly incredible: lasers instead of scalpels to cut, glue instead of sutures to close. Perhaps the next game changer is a machine capable of making diagnoses and replacing our exam—well, we are not quite there yet, but we have seen how optical coherence tomography (OCT) of the macula has changed the world of retina. It’s rare (maybe never) that a patient does not have a macular OCT performed by our retina colleagues. The use of OCT continued to evolve with the ability to image the optic nerve and give objective parameters of retinal nerve fiber layer thinning. Now, with the advent of anterior-segment OCT, we are seeing its value in our practice as well. Its impact as a game changer is already being felt with anterior-segment OCT and will continue to grow as additional uses and programs are developed
In our clinic, we utilize the RTVue spectral-domain OCT (Optovue) in several ways. For our dry eye evaluations, this device allows us to objectively measure the tear film, which helps differentiate between aqueous deficiency and other causes of symptoms. Although a slit-lamp examination may determine that, it does not give objective parameters that we can use for diagnosis, progression, and treatment response in dry eye medications and conjunctivachalasis cautery.
Across the hall, our glaucoma colleagues utilize the RTVue in a variety of ways as well. They image their narrow-angle patients as a source of diagnosis, treatment response, and patient education. OCT does not replace gonioscopy, but a picture is worth a thousand words and the objective measurements have created a new scale of narrow angle discussions. We utilize the visualization of the anterior chamber to determine tube shunt position, especially in the setting of an endothelial graft or a patient with corneal decompensation. Lastly, new OCT programs are able to image bleb morphology and correlate it to response.
In our cataract and refractive practice, we utilize the OCT for our tough calculations for patients with prior myopic LASIK; it provides another tool to determine the best lens in these difficult eyes. In the cornea space, we image patients who have corneal scarring, corneal ectasia, lamellar grafts, and more. Even more exciting are some of the new diagnostic uses, including determination of demarcation line after corneal collagen crosslinking, evaluation of ultra-thin lamellar, and even studying the epithelium in various diseases.
OCT was a game changer in retina, and it is becoming a game changer in anterior segment surgery as well.
Sumitra S. Khandelwal, MD, is an Assistant Professor of Ophthalmology at Baylor College of Medicine, and she trains residents and corneal fellows at Michael E. DeBakey Veterans Affairs Medical Center and Ben Taub General Hospital in Houston. Dr. Khandelwal states she has no financial interest in the products or companies mentioned. She may be reached at (713) 798-5143; skhandel@bcm.edu.
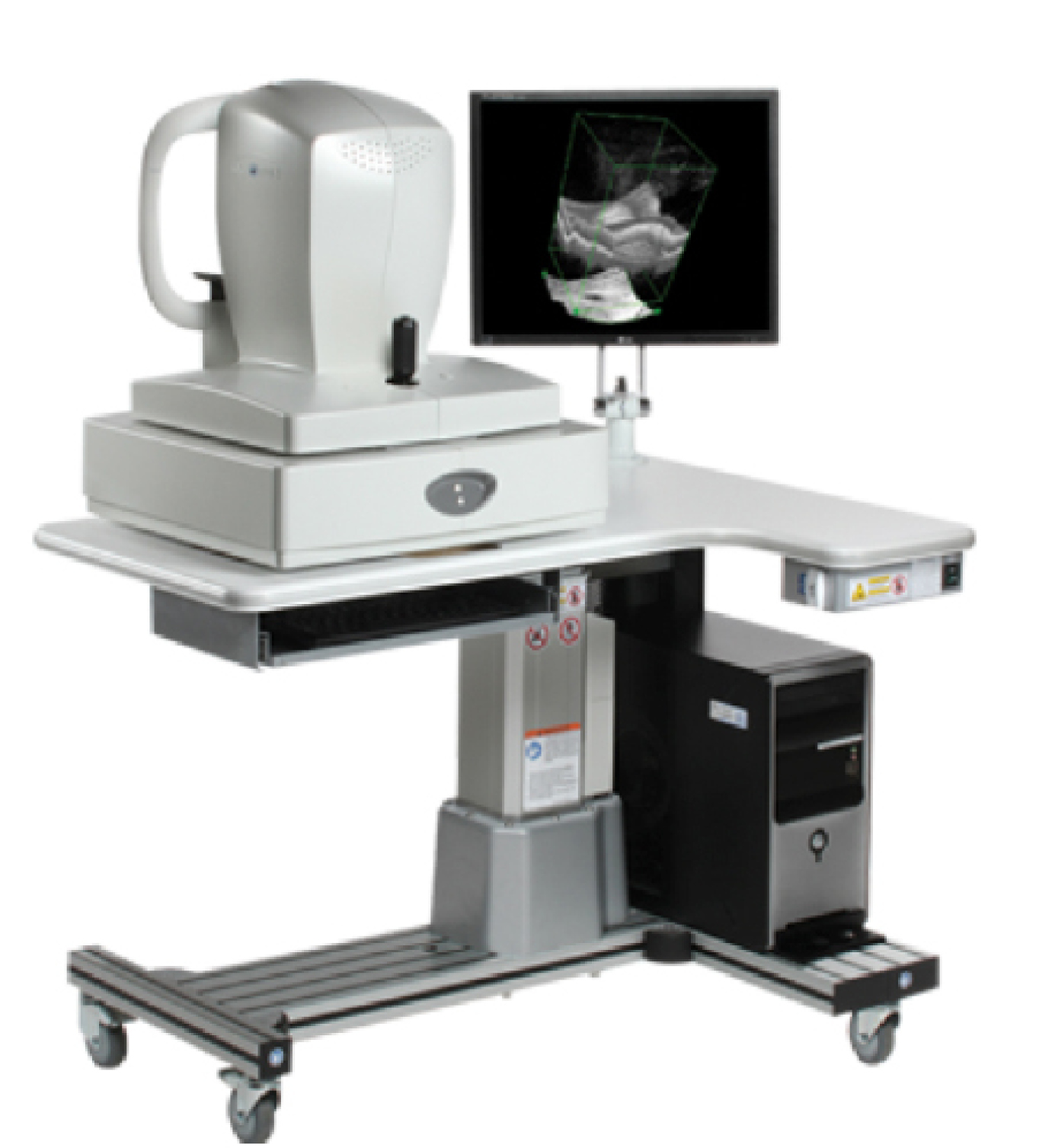
The RTVue spectral-domain OCT for retinal and corneal imaging.

Gary Wortz, MD
The Ketamine Plus Regimen
The biggest game changer I’ve experienced in the operating room in years is the Ketamine Plus regimen: the use of low-dose ketamine in conjunction with midazolam (Versed; Roche) and fentanyl in conscious sedation for cataract surgery. This regimen was developed by a select group of forward-thinking ophthalmologists, and I have implemented it with great success.
Generally, patients are given tetracaine drops in the operative eye and an intravenous dose of 50 mcg fentanyl, 1 mg midazolam, and 5 mg ketamine. The effect produces more profound sedation than even increased doses of fentanyl and midazolam, without the respiratory depression. The dose of ketamine can be repeated after 5 minutes or so if the effect was suboptimal. Typically, though, if additional sedation is needed, I give midazolam in 1-mg increments until the patient is sufficiently relaxed. The onset of effect is quick, and the duration lasts long enough to get through surgery, with limited lingering effects in the post-anesthesia care unit or after discharge.
The use of topical anesthesia with tetracaine and conscious sedation with midazolam and fentanyl is almost ubiquitous in cataract surgery. While these drugs are generally well tolerated in small doses, their effects are highly variable, especially in elderly patients with varying degrees of relative tolerance. Age, weight, alcohol use, benzodiazepine use, opioid use, and relative hepatic and renal function are pertinent variables in titrating the dose. Giving increased doses of midazolam, especially in the elderly, can cause agitation and disorientation that can be difficult to manage. The very short time period of cataract surgery and turnover time between cases leaves little time to titrate medicines. Under-sedation or over-sedation leads to anxiety for patients and physicians alike. However, there are times that physicians are left to perform a highly delicate procedure under suboptimal conditions.
With Ketamine Plus, I have seen a dramatic improvement in patient comfort and cooperation during cataract surgery. I tend not to get patients complaining that they were more awake during the second surgery (something that seemed to be a perplexing phenomenon with just fentanyl and midazolam). I have also been able to eliminate giving patients a low dose of oral diazepam (Valium; Roche) upon arrival. Oral diazepam is a smooth benzodiazepine; however, it has an extremely long half-life and can sedate elderly patients for days in some cases. With Ketamine Plus, they have almost no lingering effects upon discharge.
Ketamine in low doses (5 to 10 mg) combined with midazolam and fentanyl does not tend to cause the adverse effects that are classically described when used alone. Specifically, nystagmus and emergence phenomenon have not been an issue. However, if used in isolation and/or in larger doses, ketamine would likely be a poor choice for cataract surgery. Regardless, I would encourage cataract surgeons to try this regimen with an experienced anesthesia provider if they are looking to improve patient comfort and sedation.
Gary Wortz, MD, is an ophthalmologist at Commonwealth Eye Surgery in Lexington, Kentucky, and the Founder and Chief Medical Officer of Omega Ophthalmics. Dr. Wortz states that he has no financial interest in the products or companies mentioned. He may be reached at gary@omegalens.com.

Cary M. Silverman, MD, MBA
Femtosecond Laser Cataract
New technology pervades every area of our lives; some of it saves us time, some of it improves the precision with which we do our jobs. My game-changing technology is the femtosecond laser for cataract surgery because it has allowed me to achieve dramatically better results for my patients.
When my ambulatory surgery center (ASC) partners and I acquired the Catalys Precision Laser System (Abbott Medical Optics) nearly 2 years ago, we were the first ASC in New Jersey to adopt this technology, and we didn’t really know what to expect as far as results and expectations. I have found it completely rewarding in every way. The femtosecond laser has taken very difficult cataract cases and made them simple; it has made it fun to go into the operating room again. While high-quality premium IOLs have improved the quality of life for many patients, the femtosecond laser has actually made cataract surgery a safer, better procedure.
Our ASC now treats more patients than ever before, with several new doctors joining our ASC specifically for access to the femtosecond laser. While I have been overwhelmed at how many of our surgeons at the ASC are accepting of this technology, I also believe I could have purchased the laser alone and still benefitted financially. Because I have no doubt that the femtosecond laser allows for a better, safer procedure, I am easily able to convey my conviction to my patients. I currently perform 70% of my cataract procedures with a femtosecond laser, and I have new patients who come to our office specifically seeking femtosecond laser cataract surgery.
The current cataract population has higher expectations than ever before, in large part due to the penetration of LASIK surgery. We are finally able to offer our patients a superior result for something that is of utmost importance to their quality of life: their sight. Improved IOL technology, intraoperative aberrometers, and other technologies are all contributing to this, but the femtosecond laser is the pinnacle.
Cary M. Silverman, MD, MBA, is the Medical Director of EyeCare 20/20 in East Hanover, New Jersey. Dr. Silverman acknowledged no financial interest in the products or companies mentioned herein. He may be reached at csilverman@eyecare2020.com or via Twitter @TheLASIKdoc.

For Dr. Silverman, the femtosecond laser has made difficult cataract cases simpler to manage and has made it fun to go into the operating room again.
