A novel device in the early stages of development is taking a new approach to reducing IOP in glaucoma. The so-called electroceutical device looks like a normal pair of eyeglasses but is modified to contain a coil. The coil produces a magnetic field that generates a current. The current flows through the ciliary muscles, and the transfer of energy provides the mechanism that results in lower IOP, according to the developers and early investigators.
The technology is being developed by a company called Bionode, a startup founded by Murray I. Firestone and affiliated with Purdue University in West Lafayette, Indiana. It is the brainchild of Pedro Irazoqui, PhD, a professor of electrical and computer engineering and biomedical engineering at Purdue; glaucoma specialist Gabriel Simon, MD, of Barcelona, Spain; and Simon John, PhD, of the Jackson Laboratory in Bar Harbor, Maine.
“What we’re proposing here is a device that’s as simple as wearing a pair of glasses that will treat IOP as effectively or more effectively than eye drops, and quicker,” Prof. Irazoqui said. “In animals, we have demonstrated that it is fully reversible. So, as far as we can tell, this is safe, side-effect free, and quick-acting.”
After seeing promising data in animal models, the Bionode researchers built a human-sized version of the device, and co-inventor Dr. Simon has evaluated its use in humans. “So far, our findings show that we consistently see a change in IOP of 3 to 6 mm Hg in 10 to 15 minutes,” Prof. Irazoqui said.
A UNIQUE APPROACH
The approach is unique because of how the device works in tandem with the biological processes, Prof. Irazoqui said. “Our device regulates IOP naturally,” he explained. “The technology uses electrical signals to work with the body to restore and control physiological production and drainage of fluid into and out of the anterior chamber of the eye.”
Prof. Irazoqui serves as Chief Technology Officer of Bionode. The device originated in Purdue’s Center for Implantable Devices, where it evolved over the past 4 years through various iterations in animal testing. The company’s next goals include completing a multicenter clinical trial and applying for regulatory approvals in Europe and the United States.
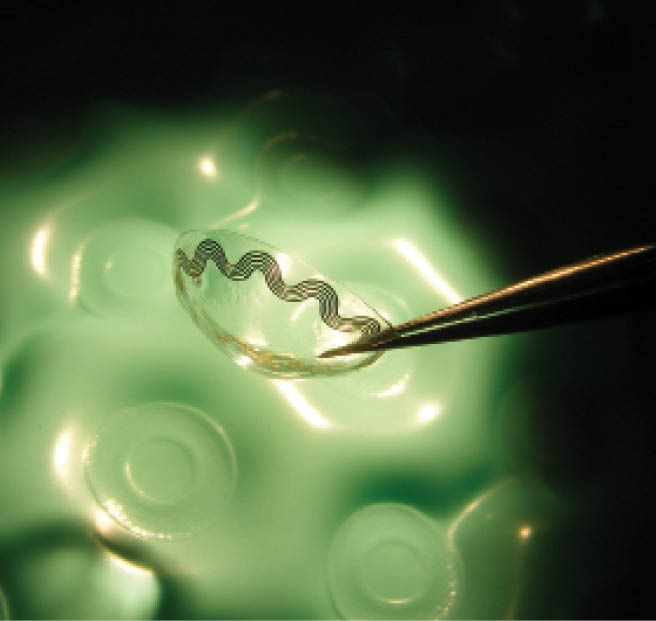
Figure 1 | A contact lens modified with a coil.
Early research included a series of experiments in a variety of animal models using the glasses either with a contact lens similarly modified with a coil to generate electromagnetic fields (Figure 1) or without the contact lens (Figure 2). The original design relied on using the glasses in tandem with the contact lens. However, Prof. Irazoqui said, “When we discussed the device with ophthalmologists, we heard that older people are often uncomfortable wearing contact lenses because less tears are produced as people age. Given that most glaucoma patients are older, we thought we might run into a compliance issue if the device was dependent on contact lens use. That put pressure on us to come up with an engineering solution that would allow us to get the same effect—the same current in the same structures—without the contact lens,” he explained. Once that was accomplished, he said, “We found that we can essentially enhance the therapy with the contact lens, but that we can get a reliable effect even without it, using the glasses alone.”

Figure 2 | Professor Irazoqui wearing prototype glasses.
MULTICENTER CLINICAL TRIAL
A clinical trial of the device is planned, with 30 patients to be recruited at each of three investigative centers: the Prism Eye Institute in Toronto, Canada; the Eugene & Marilyn Glick Eye Institute in Indianapolis, Indiana; and the Instituto Oftalmologico Gabriel Simon in Barcelona, Spain. The trial will aim to answer two pivotal questions: (1) how long does the IOP-lowering effect last, and (2) how well does the device work in a controlled population of humans with defined inclusion and exclusion criteria?
“We expect to confirm the effects that we’ve seen in our preliminary studies and begin to answer questions about durability and longevity,” Prof. Irazoqui said. “We have protocols at various stages of submission and review, and our hope is to start the multisite clinical trial by the end of the year.”
ENTHUSIASTIC INVESTIGATORS
Iqbal Ike K. Ahmed, MD, FRCSC, who will be an investigator in the clinical trial at Prism Eye Institute, has been enthusiastic about the novel technology since its inception and has referred to it as a potential game-changer.
“The preliminary findings are promising,” Dr. Ahmed said. “It’s noninvasive, and, based on early findings and in terms of theoretical possibilities, it does not seem to be of significant risk. I think the ability to acutely lower pressure but also potentially have a modulatory function where we may actually have more longer-term control is very exciting.”
“The next steps are basically to enter human trials and to evaluate the outcomes in terms of optimizing the parameters for which this will work,” he continued. “I think it has great potential. It addresses adherence, it’s noninvasive, and it has a high degree of safety. Depending on what the efficacy looks like, I think it has a promising role for all types of glaucoma.”
IOP-Lowering Goggles
Another novel IOP-lowering technology that relies on external eyewear is in development by Equinox, an early-phase medical startup company headed by glaucoma specialist John P. Berdahl, MD.
“We are developing goggles that change the pressure in front of the eye to drive the pressure inside of the eye,” Dr. Berdahl explained. He invented the device and is shepherding it through the development process.
He sees the goggles fitting into the glaucoma treatment paradigm as an adjuvant to existing therapies to help lower IOP in difficult situations. “We think that this may allow treatment of some challenging glaucomas, such as normal-tension glaucoma and severe glaucoma, where other therapies don’t work as well, and we think we can do it in a safe noninvasive way,” he said.
Dr. Berdahl is a supporter of the theory that glaucoma is a two-pressure disease and that low cerebrospinal fluid pressure may play a role in the progression of glaucoma. “If it turns out that glaucoma is a two-pressure disease—involving an imbalance between IOP and intracranial pressure—the idea would be that with the help of these goggles we gain control of the IOP portion of the equation so that we can balance those two pressures out,” he explained.
Gabriel Simon, MD, Director of the Instituto Oftalmologico Gabriel Simon, will be the principal investigator for the trial at that site. “The use of pulsed and variable electromagnetic fields that modulate the neuronal response is not a new concept, but its relation to the modulation of aqueous humor production by the ciliary body is a novelty,” Dr. Simon said. One of the aims of the clinical study, he said, is to ensure that the treatment is easy for patients to use, “so that it does not represent a drawback in their daily life.” If clinical trials show that the device can sustain the reduction of IOP, the technology might eventually be incorporated into the structure of an IOL, “and thus obviate the need to wear a contact lens,” he said.
Prof. Irazoqui said he sees the Bionode device as an ideal option for newly diagnosed glaucoma patients. “It’s not so much a question of displacing other therapies as saying that patients might want to try this first because all they have to do is wear a pair of eyeglasses. If they need more, or if they need something else as time goes on, then they can move on to other treatments.”



