Corneal transplantation is one of the most common and successful tissue transplantation procedures. However, corneal graft rejection remains a known complication, occurring after both penetrating and lamellar keratoplasty, albeit at lower rates today than in the past.1 Despite the immune-privileged status of the cornea, corneal allograft rejection remains the leading cause of corneal graft failure and occurs secondary to immune-mediated mechanisms that drive host sensitization to donor tissue.2 Other notable risk factors for graft rejection include prior graft failure (with rejection rates as high as 40% in eyes with two or more previous grafts), pre- or postoperative corneal neovascularization affecting two or more quadrants, prior use of glaucoma medications or history of glaucoma surgery, presence of anterior chamber hardware or peripheral anterior synechiae, and preexisting ocular inflammation.3
Advances in surgical techniques, improved donor tissue management, prompt recognition of signs and symptoms of rejection, and rapid initiation of therapy have all improved graft survival rates. This article discusses the tools available to the corneal specialist for when he or she encounters an episode of graft rejection.
TREATMENT OPTIONS
In general, many episodes of corneal graft rejection can be reversed if therapy is initiated upon early recognition and administered aggressively, with rates of reversal reported to be as high as 50% to 94%.4,5 The mainstay of therapy is corticosteroids, which can be administered via topical, periocular, or systemic (oral or intravenous) approaches. Recently, the role of steroid-sparing agents in the treatment and prevention of graft rejection has also increased. The optimal route of administration is not clearly defined, and diverse approaches are employed, depending on clinician experience and preference as well as on the needs of the patient.6-9
TOPICAL STEROID THERAPY
Based on surveys of Cornea Society and Castroviejo Society members conducted in 1989, 1992, and 2011, the first-line therapy for most corneal specialists facing acute graft rejection continues to be the administration of topical steroid preparations, commonly prednisolone or dexamethasone.7,8 Topical steroids are applied frequently (often hourly) throughout the day and night at the onset of the rejection episode, and they are subsequently tapered gradually over several weeks. Topical difluprednate, dexamethasone, fluorometholone, and loteprednol are used as well. Practice patterns show that, for the prevention of graft rejection, difluprednate is commonly used in high-risk grafts during the first 6 months, whereas fluorometholone and loteprednol are used after 6 months in low-risk and/or phakic eyes.6
With application of any topical steroid therapy, cataract formation and IOP elevation must be monitored by the physician. It is also important to keep in mind that frequent instillation of topical drops can limit patient adherence to the medication regimen. In the face of adverse effects or poor patient compliance, alternative therapies such as those described below may need to be considered.
SYSTEMIC STEROID THERAPY
Systemic corticosteroid therapy can be used alone or in combination with topical steroid therapy.9 Oral corticosteroids such as prednisone are prescribed at daily doses of 40 to 80 mg over the first several days and subsequently tapered based on clinical response. Intravenous corticosteroids such as methylprednisolone can also be administered, typically at pulse doses of 500 mg.9
When comparing oral and intravenous steroid therapy, Hill et al10 found a statistically significant higher graft survival rate in patients with endothelial graft rejection treated with hourly topical prednisolone acetate 1% and a single pulse dose of intravenous methylprednisolone 500 mg administered within 8 days of presentation, compared with patients who received combined topical steroids and oral prednisone in the acute rejection phase (92.3% vs 54.5%). In addition, Hill’s follow-up study did not show a significant benefit to receiving a second pulse dose of intravenous methylprednisolone 24 hours after the initial dose to reverse graft rejection.11
Important considerations when administering systemic steroid therapy include (1) patients’ abilities to tolerate high doses of corticosteroids for several weeks without adverse systemic side effects and (2) access to a hospital facility for administration of intravenous corticosteroids upon recognition of a rejection episode.
LOCAL STEROID INJECTIONS
Practice patterns show that local injections of corticosteroids are typically employed in addition to topical therapy in the context of definite graft rejection or in higher-risk grafts.6 Subconjunctival or sub-Tenon injections of dexamethasone, betamethasone, or triamcinolone can be administered alone or in conjunction with topical and oral therapy.5 Reports have also described the successful use of intracameral injections of triamcinolone and dexamethasone in reversing corneal graft rejection.17,18 These medications can be administered intravitreally as well.19
Interestingly, Costa et al5 showed that, in 16 patients who received a subconjunctival injection of 20 mg of triamcinolone and hourly topical steroid therapy during an acute graft rejection episode, there was a statistically significant reversal of graft rejection at 1 year and an improvement in BCVA, compared with patients treated with 500 mg of intravenous methylprednisolone and hourly topical steroid therapy.5 You et al19 showed that treatment with an intravitreal injection of triamcinolone (0.4 mg/0.1 mL) in conjunction with conventional therapies (topical and oral steroids and systemic cyclosporine A) compared with conventional therapies alone did not show significant differences in final visual acuity or recurrence rates; however, time to clinical improvement was significantly faster in patients treated with intravitreal triamcinolone (mean improvement in 9 days with triamcinolone therapy vs 15 days with conventional therapies alone).
During episodes of acute graft rejection, we typically use subconjunctival dexamethasone injections along with frequent topical steroid therapy if the patient has a history of steroid-related IOP elevations or advanced glaucoma. In patients who do not have a history of IOP elevation and who are pseudophakic, we administer subconjunctival triamcinolone and topical steroids as the first-line therapy.
In light of improvements seen with intravitreal triamcinolone, there are now reports in the literature documenting the reduction of graft rejection with the off-label use of an intravitreal dexamethasone implant (Ozurdex, Allergan).4,20-22 This implant provides sustained delivery of 0.7 mg of preservative-free dexamethasone to the vitreous cavity and retina for up to 6 months. Of the six treated eyes reported on in the literature, two eyes received the implant immediately at the time of graft rejection; three eyes had failed prior topical, subconjunctival, and systemic corticosteroids; and one eye showed favorable response to intravitreal triamcinolone but could not tolerate frequent injections. Resolution of graft rejection was noted by 3 months of treatment in all cases. Although five of the cases showed persistence of the treatment effect at 6 to 12 months of follow-up,4,21 one patient showed recurrence of rejection after dissolution of the implant at 3 months.20 Notably, no instances of IOP elevation or cataract formation were noted in these cases.
Selection of the appropriate patient candidate for this implant should be done in consultation with a retina specialist. Overall, these reports demonstrate the potential of the dexamethasone intravitreal implant in the treatment of corneal graft rejection, particularly in cases of poor medication compliance, poor tolerance of systemic immunosuppressive therapy, or lack of response to conventional treatments (Figure 1).
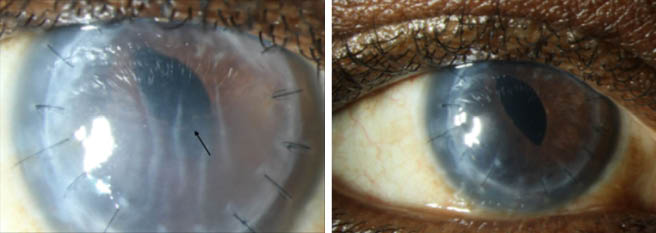
Figure 1 | A slit-lamp photograph of a 63-year-old man with a corneal scar in the right eye secondary to keratoconus, who underwent penetrating keratoplasty with pars plana vitrectomy and received an iris-sutured IOL (A). The patient sustained two episodes of graft rejection in his corneal transplant, as demonstrated by the presence of corneal edema and a Khodadoust line (black arrow). He could not tolerate the systemic side effects of oral prednisone and tacrolimus and could not receive regular intravitreal triamcinolone injections despite positive responses to intravitreal steroid therapy for his graft rejection. He underwent off-label intravitreal injection of a 0.7-mg dexamethasone implant to reduce the frequency of intravitreal steroids and preserve his corneal graft. At 1 month after the injection (B), the patient’s corneal graft cleared, and his vision returned to baseline (BCVA 20/150). He continues to receive intravitreal injections of this implant every 4 to 6 months and has done well, with no elevations in IOP.
(Images and case courtesy of Anat Galor, MD)
STEROID-SPARING TOPICAL DROPS
In a certain subset of patients, steroid-sparing topical agents can be used alone or in combination with topical or systemic corticosteroids for the treatment and prevention of corneal graft rejection. These immunosuppressive agents include topical cyclosporine and tacrolimus.
A study of pediatric patients with high-risk corneal grafts showed that the use of topical cyclosporine 2% combined with topical corticosteroids yielded statistically significant rejection-free graft survival rates of 88.9%, compared with 38.5% in patients treated with topical corticosteroid therapy alone (P = .0465); however, graft survival rates (88.9% with topical cyclosporine and corticosteroids vs 46.2% with topical corticosteroids alone) were not statistically significant.12 In a study of adult patients with high-risk transplants, the use of topical cyclosporine 2% as an adjunct to topical corticosteroid therapy again showed statistically significant higher rejection-free graft survival rates (69.7% with topical cyclosporine with corticosteroids vs 45.9% with topical corticosteroids alone); however, no significant differences in the graft survival rates were observed.13
Interestingly, a randomized controlled trial of 78 cases of high-risk keratoplasty treated with topical cyclosporine 2% drops did not show statistically significant differences in the occurrence of graft rejection. Yet, reversal of rejection was higher in patients treated with topical cyclosporine than in controls.14 These results shed light on the utility of topical cyclosporine for the prevention of graft rejection, especially in pediatric patients with high-risk transplants susceptible to graft compromise.
Similarly, a retrospective comparison of topical tacrolimus 0.03% administered with topical prednisolone 1% for the treatment of post–chemical burn high-risk keratoplasty showed statistically greater graft preservation with combined therapy (19.4% failed grafts) versus topical prednisolone therapy alone (44.4% failed grafts).15 When considering normal-risk transplants, a prospective study of 20 patients treated with topical tacrolimus 0.06% in conjunction with topical prednisolone acetate and systemic fluocortolone showed that 100% of patients treated with combination therapy remained rejection-free compared with 84% of patients treated with steroids alone.16 These studies suggest that topical tacrolimus can have a role in rejection prophylaxis in both normal and high-risk transplants.
Notably, no adverse effects were observed with topical cyclosporine. However, topical tacrolimus at a concentration of 0.06% has been associated with local discomfort, prompting cessation of therapy.16 No side effects have been noted with tacrolimus 0.03%.
SYSTEMIC ALTERNATIVES TO ORAL OR TOPICAL STEROID THERAPY
When long-term corticosteroid therapy poses severe ocular and systemic side effects, systemic agents such as cyclosporine, tacrolimus, and mycophenolate mofetil can be used, specifically in high-risk transplants. Although these agents can augment graft survival, they can also have hematologic, renal, and hepatic side effects, and patients are typically comanaged by rheumatology. Ongoing translational studies of biologic agents including monoclonal antibody therapy are showing promising results in the treatment of immune-mediated allograft rejection.23
CONCLUSION
Ultimately, the goal of treating corneal graft rejection is to preserve the graft, avoid additional surgical intervention, and maintain visual function. Although there is no defined treatment algorithm, clinicians should be aware of the various therapeutic modalities they have access to when faced with an acute graft rejection episode.
Topical, subconjunctival, and systemic steroid therapies are well-accepted treatment modalities; however, the use of intravitreal steroid therapy, notably intravitreal steroid implants, has shown emerging promise in reversing graft rejection. Larger, future studies assessing the effectiveness of this approach for corneal graft rejection could shed light on which patients are the ideal candidates and on the long-term effects of this therapy. Topical and systemic steroid-sparing agents remain treatment options for the prophylaxis of graft rejection, particularly in high-risk transplants.
1. Thompson RW Jr, Price MO, Bowers PJ, Price FW Jr. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110(7):1396-1402.
2. Maumenee AE. The influence of donor-recipient sensitization on corneal grafts. Am J Ophthalmol. 1951;34(5 2):142-152.
3. Inoue K, Amano S, Oshika T, Tsuru T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scand. 2001;79(3):251-255.
4. Vinciguerra P, Albe E, Vinciguerra R, et al. Long-term resolution of immunological graft rejection after a dexamethasone intravitreal implant. Cornea. 2015;34(4):471-474.
5. Costa DC, de Castro RS, Kara-Jose N. Case-control study of subconjunctival triamcinolone acetonide injection vs intravenous methylprednisolone pulse in the treatment of endothelial corneal allograft rejection. Eye (Lond). 2009;23(3):708-714.
6. Kharod-Dholakia B, Randleman JB, Bromley JG, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns of the Cornea Society (2011). Cornea. 2015;34(6):609-614.
7. Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004). Cornea. 2006;25(3):286-290.
8. Rinne JR, Stulting RD. Current practices in the prevention and treatment of corneal graft rejection. Cornea. 1992;11(4):326-328.
9. Panda A, Vanathi M, Kumar A, Dash Y, Priya S. Corneal graft rejection. Surv Ophthalmol. 2007;52(4):375-396.
10. Hill JC, Maske R, Watson P. Corticosteroids in corneal graft rejection. Oral versus single pulse therapy. Ophthalmology. 1991;98(3):329-333.
11. Hill JC, Ivey A. Corticosteroids in corneal graft rejection: double versus single pulse therapy. Cornea. 1994;13(5):383-388.
12. Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ. Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens. 2003;29(2):103-107.
13. Inoue K, Amano S, Kimura C, et al. Long-term effects of topical cyclosporine A treatment after penetrating keratoplasty. Jpn J Ophthalmol. 2000;44(3):302-305.
14. Sinha R, Jhanji V, Verma K, Sharma N, Biswas NR, Vajpayee RB. Efficacy of topical cyclosporine A 2% in prevention of graft rejection in high-risk keratoplasty: a randomized controlled trial. Graefes Arch Clin Ex Ophthalmol. 2010;248(8):1167-1172.
15. Magalhaes OA, Marinho DR, Kwitko S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: a cohort study. Br J Ophthalmol. 2013;97(11):1395-1398.
16. Reinhard T, Mayweg S, Reis A, Sundmacher R. Topical FK506 as immunoprophylaxis after allogeneic penetrating normal-risk keratoplasty: a randomized clinical pilot study. Transpl Int. 2005;18(2):193-197.
17. Maris PJ Jr, Correnti AJ, Donnenfeld ED. Intracameral triamcinolone acetonide as treatment for endothelial allograft rejection after penetrating keratoplasty. Cornea. 2008;27(7):847-850.
18. Reinhard T, Sundmacher R. Adjunctive intracameral application of corticosteroids in patients with endothelial immune reactions after penetrating keratoplasty: a pilot study. Transpl Int. 2002;15(2-3):81-88.
19. You IC, Yoon KC. Therapeutic effect of intravitreal injection of triamcinolone in the treatment of endothelial graft rejection: a pilot study. Cornea. 2012;31(10):1135-1140.
20. Yesilirmak N, Ozdemir ES, Altinors DD. Effect of dexamethasone intravitreal implant in a corneal graft rejection. Int J Ophthalmol. 2016;9(3):475-477.
21. Giannaccare G, Fresina M, Pazzaglia A, Versura P. Long-lasting corneal endothelial graft rejection successfully reversed after dexamethasone intravitreal implant. Int Med Case Rep J. 2016;9:187-191.
22. Cekic O, Turhan SA. Corneal Effects of the dexamethasone-releasing biodegradable intravitreous implant. Cornea. 2015;34(11):e33.
23. Jabbehdari S, Rafii AB, Yazdanpanah G, Hamrah P, Holland EJ, Djalilian AR. Update on the management of high-risk penetrating keratoplasty. Curr Ophthalmol Rep. 2017;5(1):38-48.




