In 1905, Heine published an article on the strong IOP-lowering power of his ab externo cyclodialysis procedure, which created a drainage communication between the anterior chamber and the supraciliary space.1 This procedure harnessed the power of the physiological pressure gradient between these two compartments, which has since been demonstrated to increase with rising IOP.2,3 It is generally accepted that prostaglandins analogues, a class of drugs that has become the hallmark of pharmacological glaucoma treatment, lower IOP by increasing uveoscleral outflow.4
Surgical attempts to leverage this drainage power, however, have yet to succeed owing to the difficulty of maintaining an open drainage pathway between the anterior chamber and the supraciliary space over time. Implants developed for this purpose have ceased to function because of fibrotic foreign body reactions,5 they have been taken off the market because of long-term complications,6 or they have not gained traction in surgical practice because their implantation entailed complex ab externo surgical procedures.7
As a result, reestablishing the supraciliary space as an alternative drainage route is not a part of the current continuum of glaucoma care, which recent technological innovation (eg, ab interno MIGS) has significantly expanded. Typically, pharmacological and laser-based approaches are used for early-stage glaucoma. These are followed by approaches that reestablish physiological flow through both the traditional and alternative drainage pathways and then by the use of subconjunctival stents, leaving trabeculectomy and tube shunts as the procedures of choice for advanced glaucoma.
THE PROMISE OF A BIOINTEGRATED AB INTERNO MIGS DEVICE
The implantation of the Miniject (iStar Medical) is executed as an ab interno supraciliary MIGS approach to reestablish the alternate physiological drainage pathway, analogous to trabecular meshwork–based procedures for the traditional pathway (Figure 1). The implant is 5 mm long and consists of approximately 200,000 interconnected, hollow spheres. Empty space accounts for 70% of the device’s total volume. The Miniject is open to inflow and outflow on all sides, and soft, highly flexible implant-grade silicone membranes made from Star material maintain this structure (Figure 2).
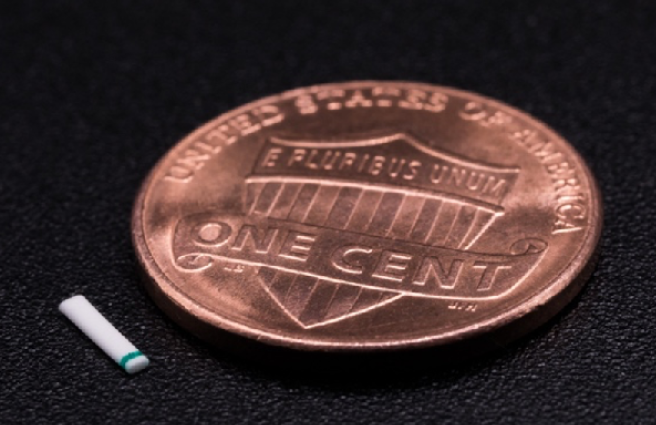
Figure 1 | The Miniject is a microinvasive technology designed to reduce IOP by optimizing the drainage pathway from the anterior chamber to the supraciliary space.
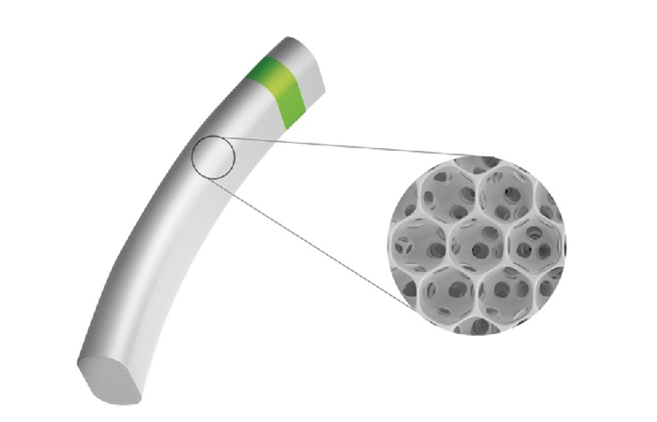
Figure 2 | The implant is 5 mm long and consists of interconnected, hollow spheres. Its innovative Star material is made of soft and flexible medical-grade silicone that conforms to the eye’s anatomy.
Preclinical data suggest that safety and efficacy are achieved through rapid biointegration after surgery. Histological analysis has shown that the hollow spheres are colonized by macrophages from surrounding ocular tissues, which does not compromise the drainage capacity. Biointegration could also explain the observation of minimal foreign body reaction and fibrosis.8
SURGICAL TECHNIQUE
A 2.2-mm peripheral temporal corneal incision is made opposite the planned implantation site. The Miniject is then advanced inside a transparent sheath through the anterior chamber and toward the iris root. A gonioprism is used for visualization, and minimal pressure is used to advance the sheath tip gently between the scleral spur and ciliary body. Positioning is correct when a green mark on the implant is flush with the ciliary spur and the device protrudes 0.5 mm into the anterior chamber, preserving a sufficient distance from the cornea. The device is released by retracting the supportive sheath (Figures 3–5).
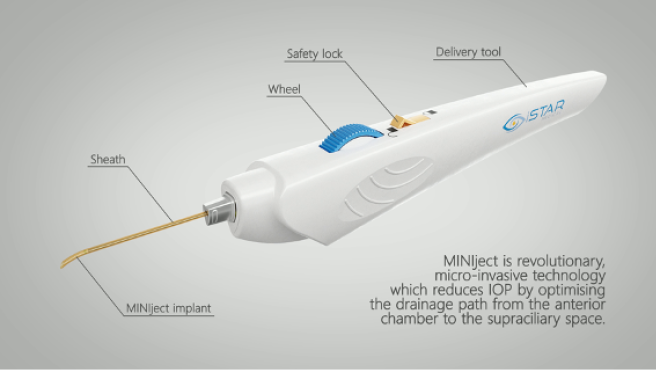
Figure 3 | The Miniject is advanced inside a transparent sheath through the anterior chamber toward the iris root and then gently positioned between the scleral spur and ciliary body. The implant is released by retracting the supportive sheath
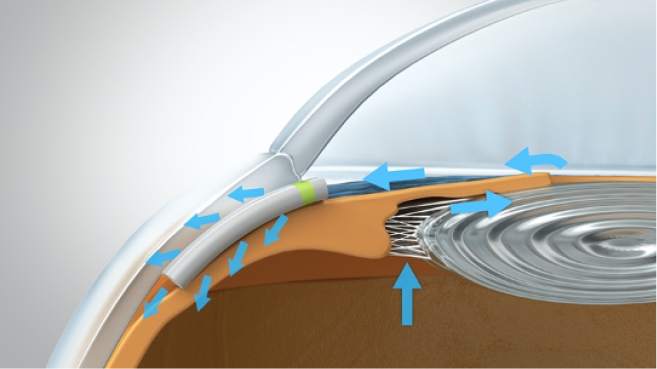
Figure 4 | The green ring on the device acts as a marker to control the implantation depth. Only 0.5 mm of the soft implant remains in the anterior chamber

Figure 5 | The final view of the Miniject in situ in the supraciliary space
All images Courtesy of iStar Medical
Typical of an ab interno supraciliary procedure, no bleb is created, and the postoperative application of an antifibrotic agent is not required.
CLINICAL EVIDENCE
In the prospective Star-I multicenter trial,9 25 patients with uncontrolled glaucoma (baseline IOP of 23.2 ±2.9 mm Hg on 2.0 ±1.1 glaucoma medications) underwent Miniject implantation as a standalone procedure. IOP decreased by 40.7% (13.8 ±3.5 mm Hg) at 24 months. Two years after surgery, patients’ need for medication was halved to 1.0 ±1.3, and approximately half of all patients were medication free. No serious ocular adverse events were observed, and further glaucoma surgery was not required by any patient. Mean central endothelial cell density (ECD) decreased from 2,411 cells/mm2 (n = 26) to 2,341 cells/mm2 (n = 21) at 24 months, which represents a 5% decrease for matched eyes. No patient had experienced a decrease in central ECD of 30% or more. In preliminary 3-year follow-up data, efficacy and a favorable safety profile were maintained.10
The Star-II study evaluated the Miniject’s safety and efficacy in a European, prospective multicenter trial that included 29 patients with medically uncontrolled open-angle glaucoma and a baseline IOP of 24.6 mm Hg despite treatment with 2.9 ±1.2 medications.11 A recent presentation of 18-month follow-up data reported a 38.5% IOP reduction to 14.7 mm Hg, and 89% of patients met the definition of a qualified success (IOP ≤ 21 mm Hg but > 5 mm Hg and ≥ 20% IOP reduction from baseline with/without glaucoma medication).12 The medication burden was reduced by 47% to 1.6 medications (n = 27), and a similarly favorable safety profile as described earlier was found. Most importantly, central ECD measurements demonstrated only a 6% decline from preoperative levels, and no patient experienced an ECD loss of 30% or more.
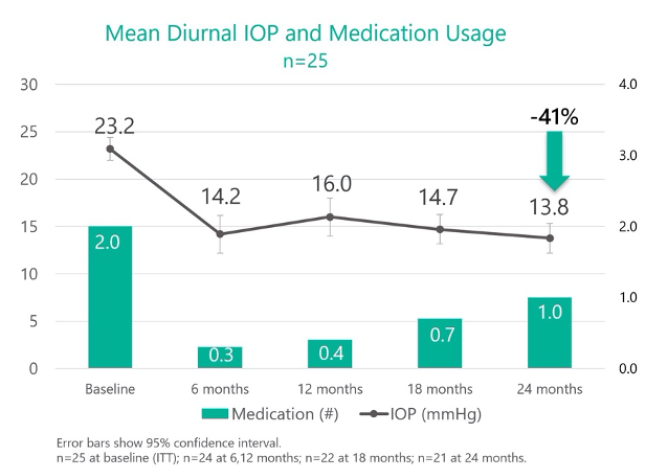
Figure 6 | In the prospective Star-I multicenter trial, 25 patients with uncontrolled glaucoma underwent implantation of the Miniject as a standalone procedure. Twenty-four months after surgery, IOP had decreased by 40.7%, and the number of medications that patients required had decreased by 50%.
OUTLOOK FOR THE MINIJECT
In clinical studies, the Miniject has demonstrated consistent, powerful, and sustained efficacy as a standalone treatment of patients with mild to moderate glaucoma and a favorable short- and long-term safety profile. Based on these findings, the FDA recently granted approval to iStar Medical to start a pivotal trial for the device. Data from the Star-V trial will be used to support a postmarket approval filing in the United States for the Miniject as a standalone procedure for the treatment of patients with primary open-angle glaucoma.13 Key findings of the Star-V trial, with a targeted enrollment of more than 350 patients, will be made available when all patients have completed 2 years of follow-up in the study.
In Europe, CE approval of the device is expected this fall, according to the manufacturer. Insofar as the Miniject would be the only therapy currently available that reestablishes the physiological drainage pathway through the supraciliary route, it will likely be used primarily as a first-line surgical treatment after pharmacological and/or laser treatment for patients with mild to moderate primary open-angle glaucoma who could benefit from significant IOP lowering. Future clinical trials may study the device’s use in combination with cataract surgery and for more advanced and complex cases.
CONCLUSION
Clinical safety and efficacy data appear to confirm promising preclinical results with the Miniject. Possibly due to biointegration of its uniquely soft and highly permeable silicone structure, inflammatory reactions to the implant remain minimal. It appears that function may be maintained for up to 3 years. Likewise, mechanical safety concerns that are sometimes described with rigid devices have not been observed with the Miniject, also possibly owing to the implant’s ability to adjust its shape to the surrounding anatomy. Further research is needed to confirm its long-term efficacy.
1. Heine L. Die Cyclodialyse, eine neuen Glaukomoperation. Med Wochenschr. 1905;31:824-826.
2. Johnson M. Unconventional aqueous humor outflow: a review. Exp Eye Res. 2017;158:94-111.
3. Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30(2):233-238.
4. Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(suppl 1):S53-64.
5. Hueber A, Roters S, Jordan JF, Konen W. Retrospective analysis of the success and safety of Gold Microshunt implantation in glaucoma. BMC Ophthalmol. 2013;13:35.
6. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass Microstent. Am J Ophthalmol. 2019;208:211-218.
7. König S, Hirneiß CW. STARflo – ein suprachoroidales drainageimplantat in der glaukomchirurgie. Der Ophthalmologe. 2018;115(8):670-675.
8. Grierson I, Minckler D, Rippy MK, et al. A novel suprachoroidal microinvasive glaucoma implant: in vivo biocompatibility and biointegration. BMC Biomedical Engineering. 2020;2:10.
9. Denis P, Hirneiß C, Durr GM, et al. Two-year outcomes of the Miniject drainage system for uncontrolled glaucoma from the Star-I first-inhuman trial. Published online October 3, 2020. Br J Ophthalmol. doi:10.1136/bjophthalmol-2020-316888 1
10. Ahmed I, Calvo E. Three-year results of a supraciliary drainage device in patients with open angle glaucoma. 9th World Glaucoma E-Congress (WGC-2021) – Abstract Book. 2021;20-21.
11. García-Feijoó J, Denis P, Hirneiß C, et al. A European study of the performance and safety of Miniject in patients with medically uncontrolled open-angle glaucoma (Star-II). J Glaucoma. 2020;29(10):864-871.
12. García-Feijoó J, Denis P, Hirneiss C, Aptel F, Lorenz K, Pfeiffer N. A European study of the efficacy and safety of a supraciliary glaucoma drainage device in patients with open angle glaucoma. 9th World Glaucoma E-Congress (WGC-2021) – Abstract Book. 2021;565-566.
13. iStar Medical receives US FDA approval to start pivotal trial for Miniject in glaucoma patients [press release]. iStar Medical. July 15, 2021. Accessed: July 23, 2021.