I have been fortunate to have been involved with CONTOURA Vision since its introduction in Europe in 2003. Once Alcon received approval by the FDA to use CONTOURA Vision in routine myopic LASIK in 2013, we were compelled by the data to revisit our primary CONTOURA Vision cases to discover a new dimension in refractive surgery’s application and measurement.
I attribute these results to the higher effective corneal normalization the procedure provides. Even eyes that we previously considered very “normal” now show even better optics in regard to the cornea vertex, which corresponds to the line of sight, as evidenced by corneal symmetry indices, contrast sensitivity measurements, and the quality of their visual image as measured with the HD Analyzer (Visiometrics).

This monograph spotlights the exceptional refractive results that my esteemed colleagues, all opinion leaders in refractive surgery, and I have been attaining with CONTOURA Vision, since the technology became the first topography-guided LASIK treatment to gain FDA approval. The following case examples demonstrate that topography-guided ablations represent an exciting new frontier in refractive corrections.
—A. John Kanellopoulos, MD, Medical Director

The newest addition to the WaveLight refractive portfolio is CONTOURA Vision (Alcon), a form of personalized laser vision correction that uses corneal topography (rather than wavefront) to reshape the cornea. In my opinion, there are three primary features that make topography-guided corneal ablations unique compared with wavefront-optimized and wavefront-guided treatments: (1) a topography-guided treatment measures and treats the aberration profile on the surface of the cornea; (2) the topography-guided treatment is centered on the apex of the cornea (or the corneal vertex), not the center of the pupil; and (3) topography-guided ablations allow the unique shape of each cornea to dictate the placement of extra peripheral pulses to blend out the treatment and minimize peripheral corneal aberrations, such as spherical aberration. These three features differentiate topography-guided corrections from wavefront-optimized corrections; the latter do not attempt to correct specific aberrations, and they use a population average for peripheral blending, as well as pupil centration. Topography-guided treatments also differ from wavefront-guided treatments, which are centered over the entrance pupil, and which do not account for peripheral corneal shape, but rather the total ocular aberrations.
Balancing the Refractive Surface
The corneal tear film is the most refractive structure in the entire eye, and most of the eye’s light-bending properties come from the anterior corneal surface that supports the tear film. If topography-guided laser vision correction can balance that refractive surface by minimizing corneal aberrations and providing a uniform aspheric curvature, it can begin to improve not only uncorrected, but best-corrected visual outcomes.1 Of course, the posterior cornea and the crystalline lens can also impart aberrations and astigmatism to the optical system, but in calculating the sphere and cylinder used to treat the eye with topography-guided ablations, we also consider the patient’s clinical refraction. Thus, when we overlay a unique topographic treatment map over an eye to custom-treat the cornea and its astigmatism, we have the opportunity to evaluate the astigmatism of the topography as well as the astigmatism of the eye’s refraction, and to titrate the best astigmatic value and axis for that treatment.
CORNEAL ABERRATIONS MAY IMPACT PERCEIVED MAGNITUDE OF ASTIGMATISM
In most eyes, the magnitude and axis of corneal astigmatism and refractive astigmatism should be close to one another, but sometimes there can be unexpected differences, and then we as ophthalmologists have to make a decision. Some clinicians say that, if there is a large disparity between the axis and the magnitude of astigmatism on the topography and the clinical refraction, then a topography-guided treatment may be contraindicated. However, I believe that higher-order aberrations on the corneal surface can impact the perceived magnitude and axis of astigmatism, although this is often not obvious, because of how the topographic map breaks down the corneal aberrations into their pure values.
For example, a significant amount of corneal coma in an eye can make it look like there is more or less refractive astigmatism, depending on its orientation, because it can only be translated in the refraction as sphere and cylinder. The refraction never tells us how much coma there is. So, the refraction has to interpret this amount based on the best fit of how that aberration would affect the refraction. The Topolyzer map (Alcon) will specify the pure cylinder magnitude and axis, and not the best-fit refractive estimate from the cornea. Therefore, it is up to the surgeon to choose this best fit for the cornea versus altering the value of cylinder to compensate for the refraction. An example of where the topographic and refractive astigmatism are vastly different, requiring an altering of the laser-treated value, is seen in the case example.
Case Example
A 24-year-old woman seeks LASIK. Surgery was performed bilaterally. The outcome for the left eye was as expected; however, in the right eye, discrepancies in preoperative cylinder measurements acquired by different mechanisms contributed to a treatment that failed to achieve the refractive target and later required an enhancement.
During preoperative testing of the right eye, manifest refraction (MR) was measured -7.50 D with +1.50 D cyl at 87° (20/20+1); cyclopegic refraction (CR) was measured -7.25 D with +1.50 D cyl at 86° (20/20-2). However, wavefront (WF) measurements were -8.78 D with +2.67 D cyl at 86° (Figure 1). Although not shown in Figure 1, a Topolyzer (Alcon) showed +2.50 D of cyl. These values for the left eye were MR -6.50 D +2.50 D cyl at 84° (20/15-2); CR -6.50 D +2.50 D cyl at 89° (20/20-2); and WF -8.07 D +3.21 D cyl at 86°. The treatment plan designed for the patient was OD: -5.50 D and -1.3 cyl at 177°; and OS: -3.75 D and -2.2 cyl at 176°.
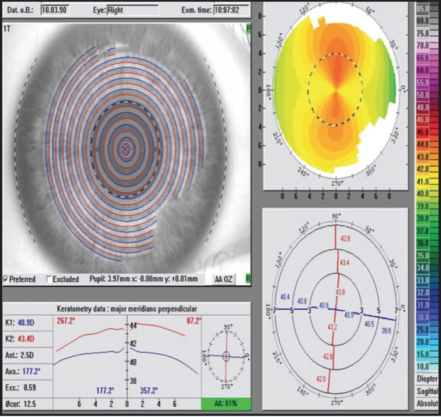
Figure 1. Baseline values OD for a patient undergoing bilateral LASIK. Note the discrepancies in cylinder measurements.
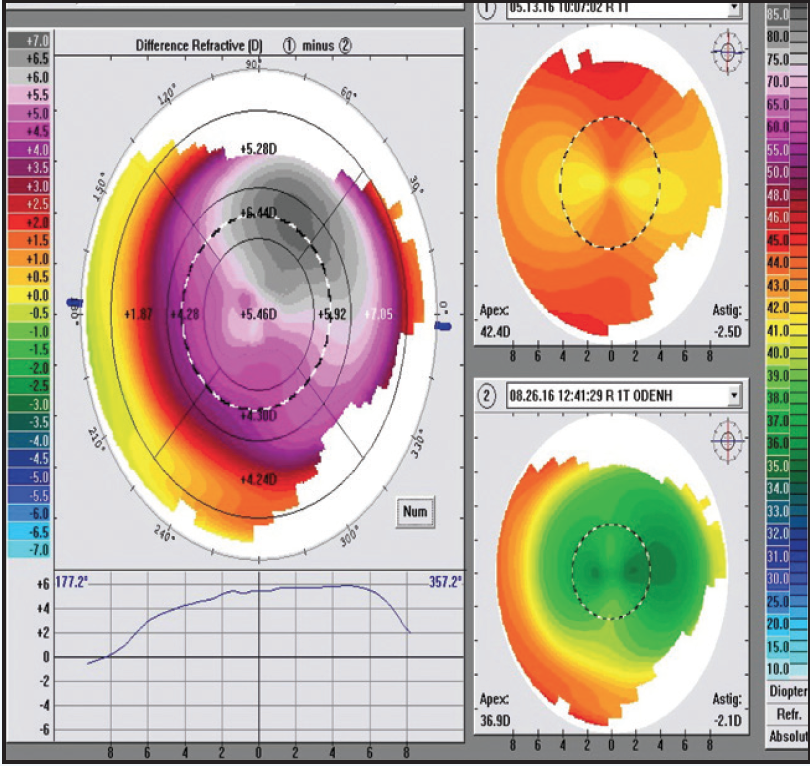
Figure 2. The target refraction was mostly achieved at the 1-week follow-up, although visual ability regressed by 3 months.
At the 1-week follow-up, 20/20 vision with a plano MR was achieved OD, although by 3 months, visual acuity was 20/30-2, MR was -1.50 D with +1.75 cyl at 98° and WF was -2.52 D with +2.13 cyl at 94° (Figure 2). The patient underwent an enhancement procedure using a laser entry of plano with -1.70 D cyl at 179° to treat the 2 lines of vision loss. At the 1-week follow-up, visual acuity measured 20/15-2, and MR was plano with +0.50 D cyl at 142°. At 3 months, visual acuity was stable at 20/15-2, MR was plano with +0.25 D cyl at 88°, and WF was -0.70 D with +0.33 cyl at 77° (Figure 3).
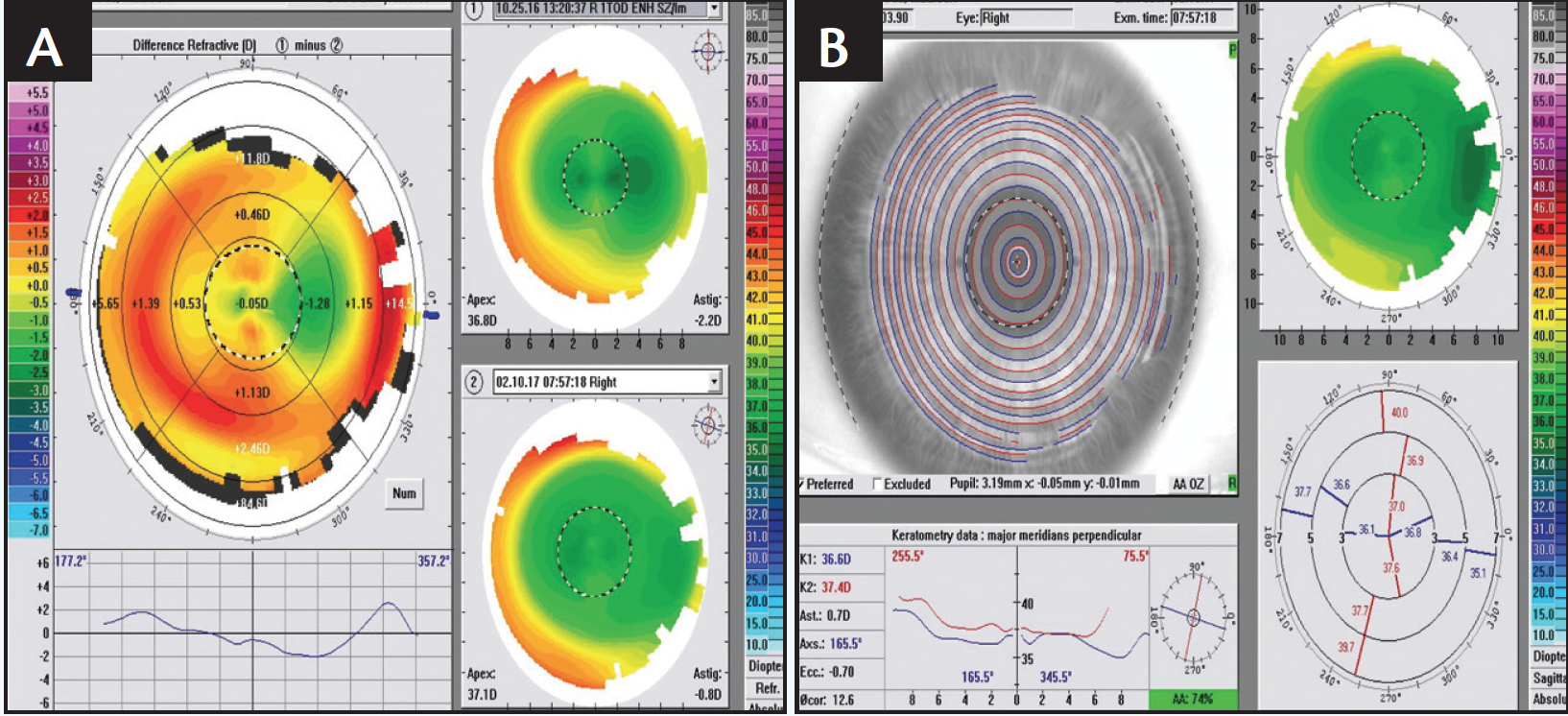
Figure 3. An enhancement procedure helped stabilize the final vision while addressing residual astigmatism (A depicts values at the time of the enhancement; B shows the results of the enhancement 3 months postoperatively).
This patient’s left eye was also treated using the manifest rather than the measured magnitude of cylinder, but because the manifest cylinder value was greater and less different from the measured, the outcome was good with 20/15 vision.
This case illustrates the benefit of treating most of the measured astigmatism, and it also shows the value of the WF in confirming when the measured value should be treated.
CONTOURA VISION ADJUSTS Q VALUE TO MAINTAIN THE PROLATE CORNEAL SHAPE
CONTOURA Vision seeks to maintain a desirable endpoint of asphericity on the cornea to preserve the eye’s prolate shape. The prolate corneal shape minimizes spherical aberration and provides a better quality of vision overall, by focusing the peripheral rays more closely with the central rays. Alignment between the periphery and the center of the cornea is important; otherwise, patients experience halos, starbursts, and other visual symptoms. Although CONTOURA Vision enables the clinician to minimally adjust the Q value, for the most part, I recommend using what the system suggests. Changing a cornea’s Q value will change the sphere, which necessitates more complex calculations in trying to be on target with emmetropia.
CONTOURA VISION TREATMENTS ARE CENTERED ON THE CORNEAL VERTEX
The location where the visual axis intercepts the cornea is very hard to define; it is somewhere between the corneal vertex and the pupillary center. Because CONTOURA Vision maps out an eye’s unique aberrations and then treats off of those maps, the corneal vertex is the appropriate place from which to base these treatments. This strategy differs from wavefront-guided treatments, which evaluate the refractive system based on rays coming through the pupil. Thus, the best placement for a wavefront-guided treatment is approximately the pupillary center.
When performing a wavefront-optimized correction, I sometimes prefer to center the treatment on or near the corneal vertex. In fact, whenever I perform hyperopic correction on an eye with a large angle kappa, where the center of the apex tends to be more toward the nasal side of the center of the entrance pupil, I find it is generally better to move toward that apex. Even when treating high myopes, I will selectively move the center of the treatment away from the center of the pupil and toward the apex in those who have a noteable angle kappa, to try and adjust centration to a distance at least two-thirds the way toward the vertex (green fixation light reflex). With CONTOURA Vision, the automatic centration is at the corneal vertex (100% of the distance from the pupillary center).
ADVANTAGES TO PERIPHERAL SHAPING WITH CONTOURA VISION
Shaping the cornea’s periphery is affected by the aberrations in the periphery and also by the extra pulses needed there in order to blend and optimize the corneal asphericity. In my clinical experience with CONTOURA Vision, I have seen some amazing postoperative topographies that show a very uniform ablation zone all the way out to the periphery in very high myopes, which I would not expect in a normal wavefront-optimized or wavefront-guided treatment. In the very first patient that I treated with CONTOURA Vision, the preoperative refraction was -8.00 D, and his vision was 20/15+2 UCVA on the first week after surgery. This patient’s postoperative topography was more beautifully symmetrical and centrally uniform (Figure 3, bottom right) in comparison to the preoperative topography (top right). The difference map (Figure 3, left) shows the customized asymmetric treatment profile, which illustrates why CONTOURA Vision works better than a pure refractive wavefront-optimized treatment. If we ophthalmologists can continue to produce such outcomes with CONTOURA Vision, I believe we will begin to directly challenge the conventional wisdom that phakic IOLs are a better modality for treating moderate-to-high myopes. Since phakic IOL procedures are more invasive and more expensive, this improved quality of laser vision correction with CONTOURA Vision begins to match the kind of quality one can achieve from an IOL, without the associated intraocular risks.
CONTOURA VISION CAN ASSIST FUTURE CATARACT SURGERY
When it does become time to replace the natural lens, which has become cataractous, the corneal optics following CONTOURA Vision are the best possible with laser vision correction. This facilitates better postoperative cataract surgery outcomes, especially when one considers implanting premium multifocal IOLs.
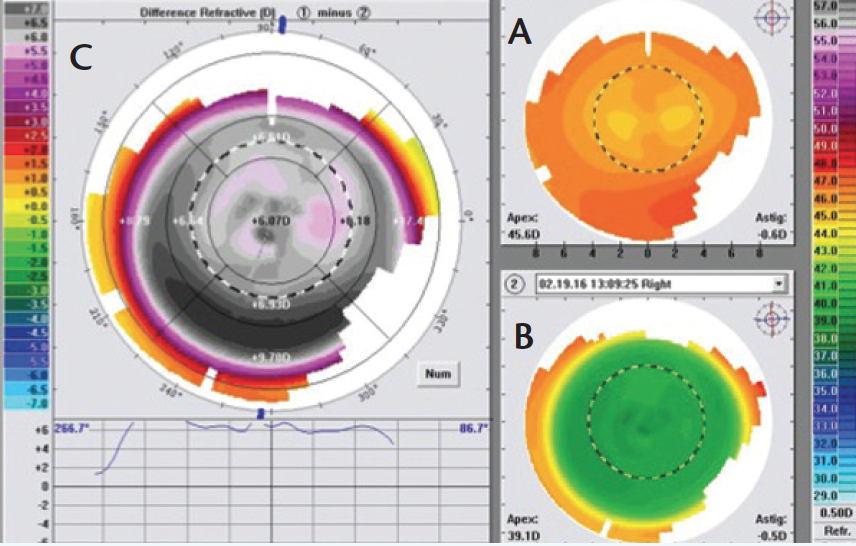
Figure 4. Dr. Krueger’s first CONTOURA Vision patient (OS). The figure shows the eye’s preoperative topography (A; upper right), 1-week postoperative map (B; lower right), and the difference map (C; left). The postoperative map (B) reveals a uniform central curvature, which is achieved by the complex prolife shape change noted in the difference map (C). This high myope achieved 20/15+ acuity in the first postoperative week.
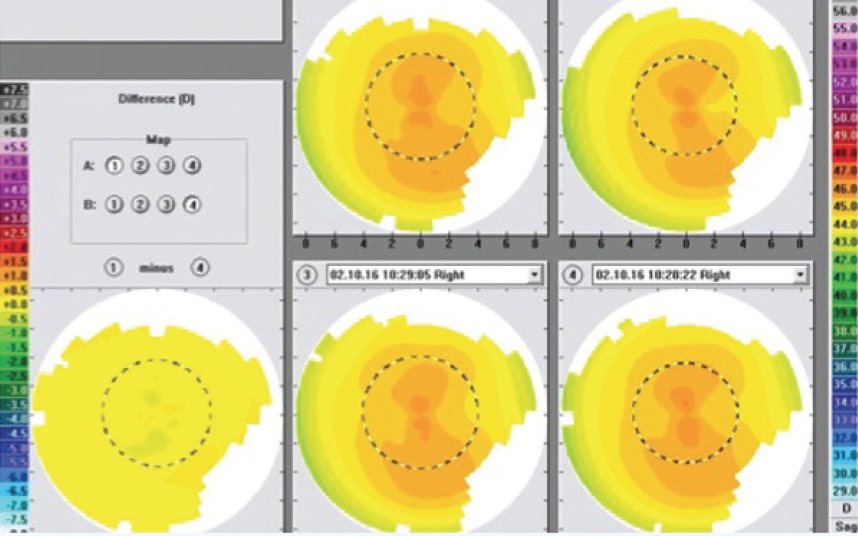
Figure 5. This figure shows the four preoperative Topolyzer maps that the author selected to create the composite topography map (from which the treatment profile was created). Their measurements are all within 0.50 D of each other across the entire difference map.
MY TYPICAL CONTOURA VISION WORKUP
As part of my CONTOURA Vision platform, I have an ALLEGRETTO Wave Eye-Q excimer laser (Alcon), which is the 400-Hz laser. My typical preparation for CONTOURA Vision surgery is as follows. The day before, I usually review my nomogram and determine my final correction for the patient based on this nomogram. Then, my technician and I look at the CONTOURA Vision maps, we select the best four that have the greatest quality and uniformly similar pattern, and from those we create an average topographical map, from which I perform the ablation. All four preoperative maps have to be within 0.50 D of each other anywhere on the cornea. The goal is to minimize differences that will show up as noise on the composite map. Figure 4 shows the four selected maps from my first high myopia patient treated in Figure 3. Each of the four selected maps are within 0.50 D of each other across the entire difference map.
On the day of surgery, I bring the composite map to the WaveLight laser, I look at the system’s proposed ablation profile, and I match this with the composite topography map and the clinical refraction. Then, I enter my final sphere and cylinder calculations into the machine. Thus, I have the ability to match up and titrate the magnitude of astigmatism and axis of astigmatism and sphere, based on what I see on the topography. If the topography map tells me there is a little more or a little less astigmatism than the refraction, then I have to use my judgment to input what I think are the best values for the magnitude and axis of astigmatism and the spherical component. I will sometimes use the preoperative wavefront map (LADARWave [Alcon]) to guide the proposed treatment profile, striving to treat the full topographic cylinder and axis. Sometimes, this confirms that I must adjust the cylinder according to the refraction and wavefront, as was illustrated in the first case example. I appreciate that CONTOURA Vision allows this level of physician input into the final correction. To me, this is the definition of an individualized treatment.
SHORTER ABLATION TIMES
Each surgeon will develop a nomogram for CONTOURA Vision over time, and interestingly, mine usually tells me to treat very slightly more than my wavefront-optimized nomogram. I believe part of the reason for this is that wavefront-optimized profiles treat all the astigmatism at the end of the ablation. As an example, highly myopic ablations will dry out the corneal tissue by the end of a wavefront-guided or wavefront-optimized ablation, and the tissue will over-respond and overcorrect if I treat the full amount of astigmatism that the refraction indicates. With CONTOURA Vision, however, astigmatism is treated at the same time as the sphere and other aberrations, so I can enter more cylinder correction into the treatment plan.
Let’s say I am treating a -9.00 +2.00 x 90 myopic astigmat. A wavefront-optimized platform will treat in minus cylinder, so the treatment is really -7.00 -2.00 x 180. Wavefront-optimized systems will treat all of the -7.00 D first, and then it will treat the -2.00 D at the end. Because they leave the astigmatism for the end, however, the tissue is dried out from the lengthy ablation and tends to over-respond to that cylinder. Thus, I usually nomogram-adjust the cylinder correction with high myopes in wavefront-optimized treatments, and in this case, I would only treat -1.25 or 1.50 D of cylinder. With topography-guided treatments, I do not have to do this, because this procedure treats the astigmatism simultaneously with the sphere.
THE UNIQUE WAY IN WHICH CONTOURA VISION SHAPES THE CORNEA
One interesting aspect of topography-guided ablations is their effect on the central corneal tissue. If I treat an eye with a low level of myopia that has a little paracentral asymmetry, portions of the postoperative topography may actually look steeper instead of flatter. Also, the topographic difference map may show regions where a negative value of corneal tissue was removed. Obviously, the ablation did not add tissue. By treating far out in the periphery, CONTOURA Vision causes central or paracentral cornea tissue to become steeper. Thus, the CONTOURA Vision procedure is doing something unique, by ablating tissue in such a way that the very flat areas of the cornea are being made steeper, while the steep areas are being flattened. This is the unique process by which CONTOURA Vision makes a uniformly prolate cornea. This effect is only made possible with an integrated system—a laser that can interpret the treatment maps and can fully compensate for that intended shape, rather than simply adding a few pulses here and there, the way wavefront-guided treatments do. The difference map in Figure 1 illustrates an example of this, where the central, green-colored, vertically oriented astigmatism represents a negative removal (addition) of tissue, due to the paracentral and peripheral treatment that steepens the center.
PATIENT EDUCATION AND SELECTION
CONTOURA Vision is fast becoming my first-line treatment for myopia up to 8.00 D with up to 3.00 D of astigmatism. I would say that 90% of my patients who qualify within a myopic range (with astigmatism of less than 3.00 D) are opting for it. Previously, wavefront-optimized ablations were my first choice.
In my discussions with patients, I tell them that the CONTOURA Vision procedure was approved based on an FDA study performed 2 years ago, in which 30% to 40% of the subjects saw better after the treatment than they did wearing their best pair of glasses, and the other 60% to 70% saw at least as well after surgery as they did with their glasses. All of my CONTOURA Vision patients thus far are happy with their outcomes, and I continue to collect data to compare my outcomes with those in the FDA trial.
1. Stulting RD, Fant BS; T-CAT Study Group. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11-18.
