The innermost layer of the cornea, the endothelium, maintains corneal clarity by serving as a barrier to aqueous humor and functioning as a metabolic pump to remove excess fluid from the stroma. A normal human endothelial cell count at birth is 4, 000 to 5,000 cells/mm2. That figure decreases progressively with age by about 0.6% per year.1 This rate of cellular decline can be compounded by damage secondary to eye surgery, trauma, or diseases of the endothelium, the most common of which is Fuchs endothelial corneal dystrophy (FECD). When cell density drops below about 500 cells/mm2, endothelial compromise results in corneal edema, clouding, and decreased vision.2
First described by Ernst Fuchs in 1910, FECD is a degenerative disease of the corneal endothelium that results in cell dysmorphia and dysfunction.3 The surgical management of FECD usually entails replacing the diseased endothelium via Descemet stripping automated endothelial keratoplasty or Descemet membrane endothelial keratoplasty. In the United States, FECD is an increasingly common indication for corneal transplantation, accounting for almost one-third of all transplants performed in 2017.4 This number is on the rise, with more than 50% of all endothelial keratoplasties in the United States performed for endothelial dystrophy in 2018, according to Patricia Dahl, CEO at The Eye-Bank for Sight Restoration in New York (written communication, February 2020).
Although the surgical management of FECD is widely accepted, these procedures are invasive, healing time is often prolonged, and complications such as allograft rejection and detachment can occur. In addition, there is a global shortage of corneal graft tissue, with only one cornea available for every 70 needed.5 Investigators around the world are therefore avidly searching for graftless treatment options for FECD.
RHO-ASSOCIATED KINASE INHIBITORS
Rho-associated kinase (ROCK) inhibitors are one promising adjunct for FECD treatment. ROCK signaling pathways are responsible for a broad spectrum of fundamental cellular events, and these pathways have become important therapeutic targets in the treatment of many pathophysiologic conditions (see Use of ROCK Inhibitors in Other Areas of Medicine).6 Cellular stresses on the corneal endothelium activate the Rho/ROCK/myosin light chain phosphorylation pathway, which decreases cell adhesion to the underlying extracellular matrix and activates the apoptosis cycle.7 ROCK inhibition suppresses myosin light chain phosphorylation, discourages apoptosis, and counteracts the loss of cell adhesion.7 In addition, topical ROCK inhibitors are potent enhancers of corneal endothelial wound healing because they promote the proliferation of residual healthy endothelial cells peripheral to the site of injury.8
The use of ROCK inhibitors in the medical management of FECD was first reported in 2013, when Koizumi and colleagues described the successful treatment of a 52-year-old Japanese man. Treatment consisted of denuding the central corneal endothelium and applying a ROCK inhibitor topically for 1 week. The patient’s bullous keratopathy resolved; the cornea remained clear at 2 years, and visual acuity was 20/20.9
In 2018, the same investigators reported successfully treating bullous keratopathy from various causes with intracameral injections of cultured human corneal endothelial cells suspended in a solution with a ROCK inhibitor. Descemet membrane was stripped from the central 8-mm–diameter area of the cornea prior to injection, and the patients remained in a prone position for 3 hours to enhance adhesion of the injected cells. Six months after the injections, corneal transparency had been restored to 100% of the treated eyes, and endothelial density was greater than 500 cells/mm2.10
DESCEMET STRIPPING WITHOUT ENDOTHELIAL KERATOPLASTY
Corneal endothelial cells have an extremely limited ability to proliferate in vivo. Instead, neighboring cells compensate for endothelial cell loss by enlarging and spreading to cover the defects.11 Since 2013, several researchers have reported observing corneal clearing after iatrogenic endothelial trauma, which gave rise to the idea of healing by primary intention as a planned surgical strategy.12-14 A procedure dubbed Descemet membrane stripping only (DSO) involves performing a descemetorhexis without transplantation of a donor graft; this is the newest proposed surgical treatment for FECD (Figure). The theory is that removing the diseased Descemet membrane can release contact inhibition and facilitate the central migration of healthier peripheral endothelial cells.15
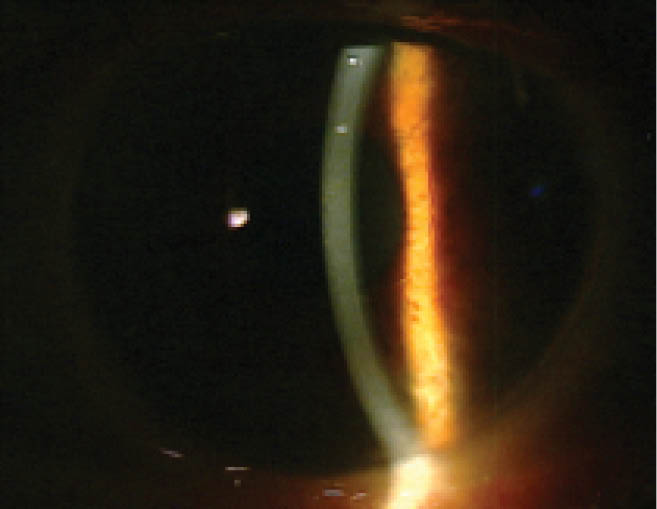
Figure. Five years after DSO.
Borkar and colleagues evaluated 13 eyes of 11 patients with FECD and no countable central endothelial cells preoperatively who underwent DSO with stripping of the central 4-mm–diameter area of endothelium. The corneas of four of these eyes cleared by postoperative month 1. Clearing occurred in another four eyes by month 3 and in two eyes by month 6. Three eyes eventually required endothelial keratoplasty.13 Iovieno et al reported clearance in four of five eyes that underwent DSO with a 4-mm descemetorhexis.16
Although it is not well understood what causes certain patients to be “fast responders, responders, slow responders, or nonresponders” after DSO—terminology coined by Kathryn Colby, MD, PhD—experience suggests that surgical technique and patient selection are two keys to successful DSO.13 When we spoke to Dr. Colby for this article, she stated that a preoperative pachymetry reading greater than 625 µm was associated with reduced corneal clearance after DSO in her initial series. Dr. Colby said she examines the peripheral endothelium to ensure that it is healthy and without significant guttae because these cells will repopulate the area of stripped cells centrally. She prefers the peripheral cell density to be greater than 1,500 cells/mm2, whereas Moloney has found a peripheral cell density greater than 1,000 cells/mm2 to be sufficient.17 The visual status of the fellow eye is another important consideration during surgical planning because an initial decline in vision after DSO is expected. Patients should be counseled preoperatively that visual recovery will be gradual and is not guaranteed.
Restricting the descemetorhexis to the central 4 mm seems to promote a higher rate of corneal clearance by limiting the surface area that the remaining endothelial cells are asked to repopulate. Initial series using a larger descemetorhexis (8 mm in a study by Bleyen et al18 and 6 mm in a study by Koenig19) were largely disappointing. Of note, increasing the descemetorhexis from 4 to 6 mm in diameter more than doubles the surface area of stripped cells (9/4 = 2.25).13
It is important to avoid surgical trauma during Descemet stripping because trauma can elicit a fibrotic scarring response that will challenge the central migration of peripheral endothelial cells. Davies and colleagues described a descemetorhexis technique that minimizes scarring to the corneal stroma: a Fogla Descemet membrane stripping hook is used to score only 2 clock hours, and the rest of the descemetorhexis is peeled using forceps (Utrata or MicroSurgical Technology).20
COMBINING DSO WITH A ROCK INHIBITOR
Moloney et al used ripasudil 0.4% (Glanatec, Kowa, not available in the United States), a ROCK inhibitor approved in Japan for the treatment of glaucoma, as a salvage treatment for eyes with corneas that did not clear within 2 months after DSO. In this study, topical ripasudil administered six times a day for 2 weeks resulted in total corneal clearance in two of 12 eyes whose corneas failed to clear completely after DSO alone.17
In a prospective controlled trial by Macsai and Shiloach, DSO patients were randomly assigned to treatment with topical ripasudil 0.4% administered four times a day for 2 months or to no treatment. The ripasudil group recovered vision more quickly and had a higher average endothelial cell density at 3, 6, and 12 months.8
FUTURE DIRECTIONS
To date, no studies have evaluated the long-term success rate of graftless corneal endothelial replacement for patients with FECD, although the index patient in the series by Borkar et al has maintained a clear cornea for 6 years since undergoing DSO, Dr. Colby told us.13 Transitioning to DSO with or without an adjunctive topical ROCK inhibitor as the treatment of choice for FECD has the potential to greatly decrease the cost of visual rehabilitation for both individual patients and society. Additional studies are needed to elucidate the important predictive factors for success with these new strategies.
1. Wilson RS, Roper-Hall MJ. Effect of age on the endothelial cell count in the normal eye. Br J Ophthalmol. 1982;66(8):513-515.
2. Bourne WM. Clinical estimation of corneal endothelial pump function. Trans Am Ophthalmol Soc. 1998;96:229-239; discussion 239-242.
3. Ernest F. Dystrophia epithelialis corneae. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1910;76:478-508.
4. Eye Bank Association of America. Eye Banking Statistical Report. Washington, DC: Eye Bank Association of America; 2017.
5. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167-173.
6. Moura-Coelho N, Tavares Ferreira J, Bruxelas CP, Dutra-Medeiros M, Cunha JP, Proença RP. Rho kinase inhibitors—a review on the physiology and clinical use in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257(6):1101-1117.
7. Okumura N, Fujii K, Kagami T, et al. Activation of the Rho/Rho kinase signaling pathway is involved in cell death of corneal endothelium. Invest Ophthalmol Vis Sci. 2016;57(15):6843-6851.
8. Macsai MS, Shiloach M. Use of topical Rho kinase inhibitors in the treatment of Fuchs dystrophy after Descemet stripping only. Cornea. 2019;38(5):529-534.
9. Koizumi N, Okumura N, Ueno M, Nakagawa H, Hamuro J, Kinoshita S. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea. 2013;32(8):1167-1170.
10. Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995-1003.
11. Sarnicola C, Farooq AV, Colby K. Fuchs endothelial corneal dystrophy: update on pathogenesis and future directions. Eye Contact Lens. 2019;45(1):1-10.
12. Moloney G, Chan UT, Hamilton A, Zahidin AM, Grigg JR, Devasahayam RN. Descemetorhexis for Fuchs dystrophy. Can J Ophthalmol. 2015;50(1):68-72.
13. Borkar D, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea. 2016;35(10):1267-1273.
14. Ziaei M, Barsam A, Mearza AA. Spontaneous corneal clearance despite graft removal in Descemet stripping endothelial keratoplasty in Fuchs endothelial dystrophy. Cornea. 2013;32(7):e164-e166.
15. Garcerant D, Hirnschall N, Toalster N, Zhu M, Wen L, Moloney G. Descemet’s stripping without endothelial keratoplasty. Curr Opin Ophthalmol. 2019;30(4):275-285.
16. Iovieno A, Neri A, Soldani AM, Adani C, Fontana L. Descemetorhexis without graft placement for the treatment of Fuchs endothelial dystrophy: preliminary results and review of the literature. Cornea. 2017;36(6):637-641.
17. Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis without grafting for Fuchs endothelial dystrophy-supplementation with topical ripasudil. Cornea. 2017;36(6):642-648.
18. Bleyen I, Saelens IE, van Dooren BT, van Rij G. Spontaneous corneal clearing after Descemet’s stripping. Ophthalmology. 2013;120(1):215.
19. Koenig SB. Long-term corneal clarity after spontaneous repair of an iatrogenic descemetorhexis in a patient with Fuchs dystrophy. Cornea. 2013;32(6):886-888.
20. Davies E, Jurkunas U, Pineda R 2nd. Predictive factors for corneal clearance after descemetorhexis without endothelial keratoplasty. Cornea. 2018;37(2):137-140.







