Presbyopia affects the large majority of people who are 45 years of age or older. The longtime mainstays of treating this natural, age-related condition are spectacles and contact lenses. Both of these modalities can be inconvenient for patients, and users can become intolerant of or develop an infection from wearing contact lenses. Surgical procedures that treat presbyopia include IOL implantation, corneal inlays, and laser procedures—all of which can be invasive and difficult to reverse.
A pharmacologic solution to presbyopia is therefore of great interest to eye care providers and patients alike. Here’s an update on agents in development.
ACCOMMODATIVE ABILITY
A youthful eye has normal protein sulfhydryl groups within the lens fiber cells that allow the lens to change shape through central displacement of cytosol. It has been proposed that, in the aging eye, oxidation-induced disulfide bonds form between crystalline lens proteins, barring the movement of cytosol and thus causing the lens to stiffen.
Previously called EV06 ophthalmic solution, UNR844 (lipoic acid choline ester [LACE] 1.5%, Novartis) works directly on the flexibility and accommodative ability of the crystalline lens. This prodrug is designed to penetrate the cornea and break down into lipoic acid and choline. Lipoic acid is then hydrolyzed by esterase in the tear film and cornea and subsequently reduced into dihydrolipoic acid within the fiber cells of the crystalline lens, which causes hydrolysis of the disulfide protein bonds. Cytosol regains the ability to flow freely, and the oxidative process may be reversed, thereby restoring the lens’ elasticity and accommodative amplitude by allowing the lens to change shape again (Figure 1).
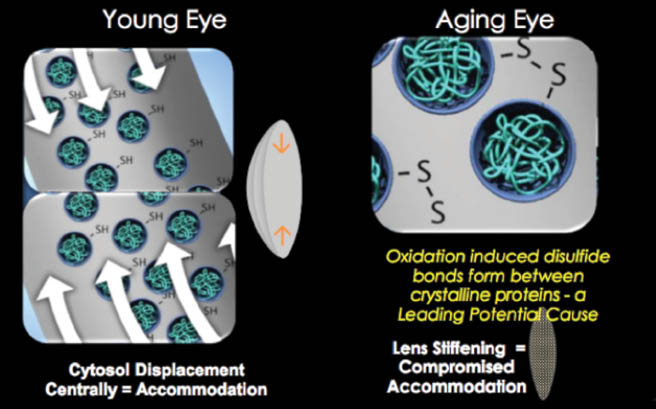
Figure 1. Depiction of why presbyopia occurs in the aging eye.
In a phase 1/2 study evaluating the safety and efficacy of EV06 ophthalmic solution 1.5%, 50 patients received one drop of the drug twice daily and 25 patients received a placebo.1 By day 91, distance-corrected near visual acuity (DCNVA) was 20/40 in 82% of patients treated with EV06 and 48% of patients who received a placebo compared to baseline values of 30% and 28%, respectively. Moreover, DCNVA was 20/32 in 68% of patients treated with EV06 and 28% of patients who received a placebo compared to baseline values of 8% for both groups. Additionally, 22% of patients experienced an improvement of 3 lines after 90 consecutive days of twice-daily treatment.
In a preclinical in vivo study, LACE was instilled three times a day in one eye of 8-month-old mice for 5 weeks. Contralateral eyes served as controls. The viscoelasticity of excised treated lenses was 40% greater than that of untreated lenses. The investigators observed a dose-dependent reduction in lens protein sulfide and a concurrent increase in lens softness.2
A phase 2 study of LACE has been completed, and the data are being evaluated. The results should be informative, as will be the FDA’s requirements for approval of this new compound.
DEPTH OF FIELD
Other presbyopia-correcting pharmacologic agents in development exert a pinhole effect to increase patients’ natural depth of field (Figure 2). I consider the five main requirements of miotics to be:
- Comfort and tolerability on instillation;
- Fast onset and sufficient duration of action;
- Modulation of the pupil to an efficient size for added depth of focus without loss of contrast or nighttime visual acuity;
- Excellent safety profile; and
- No degradation of distance visual acuity.
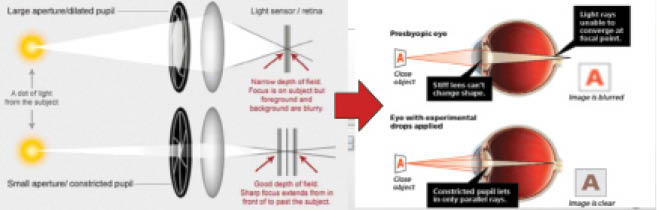
Figure 2. Several of the products in development exert a pinhole effect to enhance natural depth of focus.
Courtesy of Allergan
AGN-1883
AGN-1883 (Allergan) is a topical drop intended to increase depth of focus. It is instilled once daily in both eyes or in the nondominant eye alone. Phase 2b studies are complete, and phase 3 studies began in late 2018. This drug is reportedly well tolerated and has a fast onset of action. The company says the agent improves the quality of patients’ near visual acuity for several hours after instillation, even in mesopic conditions.
Allergan received a provisional patent for pilocarpine at a concentration ranging from 1% to 1.5% for the treatment of presbyopia. The company expects to be the first to market with a presbyopia-correcting drug that has completed FDA trials. It is targeting a US launch date in mid-2021.
PRX-100
The miotic agent aceclidine is the active ingredient in Liquid Vision (PRX-100, Presbyopia Therapies). According to the company, Liquid Vision has a 30-minute onset of action and a 4-hour duration of effect. The drug would be administered to one or, preferably, both eyes. The company is positioning Liquid Vision as a complement to rather than a replacement for other modes of presbyopic correction (eg, spectacles, contact lenses, surgery). The idea is to modulate pupillary size without increasing accommodation so that distance visual acuity is not adversely affected. This is why other products in development contain concentrations of pilocarpine lower than 2%.
In a phase 2b study, two test candidates of PRX met the primary safety and efficacy endpoints. According to the company, the drug was well tolerated, and treated patients achieved an improvement in DCNVA of 3 or more lines that lasted for several hours. Presbyopia Therapies expects to start phase 3 trials of the drug this year.
CSF-1
According to Orasis Pharmaceuticals, CSF-1 contains a proprietary combination of existing and well-studied ingredients, and the active ingredient is a low-dose pupillary miotic. The product will be administered to both eyes. A phase 2b multicenter, double-masked, repeated-administration clinical trial evaluated the drug’s safety and efficacy in 166 patients. In that study, CSF-1 reportedly demonstrated an exceptional safety and tolerability profile. Treatment achieved a statistically significant improvement (3 or more lines) in DCNVA. Importantly, patients also showed no reduction in distance vision, both at regular and low luminance (Figure 3).
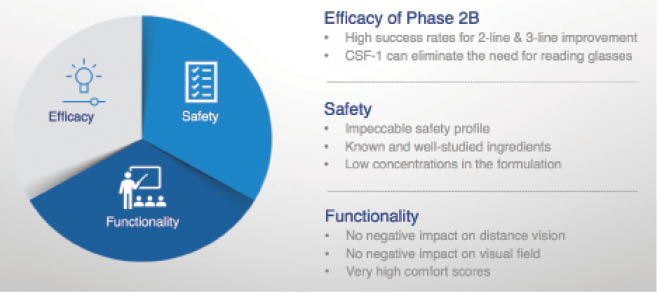
Figure 3. In a phase 2b trial, CSF-1 appears to have achieved what the FDA is looking for in a daily treatment for presbyopia in terms of safety, efficacy, and a 3-line improvement in DCNVA.
Courtesy of Orasis Pharmaceuticals
The company expects to initiate a phase 3 trial soon.
EyeFocus
EyeFocus (OSRX, an affiliate of Ocular Science) is a compounded miotic eye drop for the treatment of presbyopia that is scheduled for release in the second quarter of 2020. The product contains low concentrations of pilocarpine, phenylephrine, pheniramine, and ketorolac. It will be available in two strengths, labeled as EyeFocus and EyeFocus+.
OSRX developed EyeFocus in partnership with Luis Felipe Vejarano, MD, who introduced the presbyopia medication FOV Tears internationally in 2016. According to Dr. Vejarano, EyeFocus is similar to his original eye drop.3 He therefore expects EyeFocus to be similarly effective. Both products decrease the size of the pupil and improve accommodation at the ciliary body.
It is important to note that compounded products do not go through the regulatory pathway of FDA-approved products, which requires adequate and well-controlled studies of safety and efficacy that evaluate the entire formula as well as the therapeutic contribution of each active ingredient. It therefore may be difficult to determine the additive benefits of each ingredient in a compounded drug that contains multiple active ingredients.
An initial proof-of-concept study of EyeFocus was conducted in April 2019 on nine presbyopic patients (18 eyes) over a 2-day period. Study participants ranged in age from 44 to 64 years. They received one drop of the medication in each eye at hour 1 and again at hour 5. The following trends reportedly were observed:
- DCNVA improved by 3 lines (patient average);
- At hour 1, reading vision was J3 or 20/40 in 66% of patients;
- During hours 3 to 5, reading vision was J3 or 20/40 in 89% of patients and J2 or 20/30 in 66% to 71% of patients;
- 78% of patients maintained J3 reading vision for 8 hours;
- 11% of patients reported diminished contrast sensitivity for 2 to 3 hours after instillation of the drug; and
- 20% of patients experienced ciliary spasm after the initial dose.
CONCLUSION
Pupillary miotics will be the first pharmacologic treatments for presbyopia to market. These agents have demonstrated safety, efficacy, and ease of use in early studies. It remains to be seen which patient populations will be best suited to this form of treatment, and further research is required to demonstrate these agents’ long-term tolerability. Results thus far, however, suggest that pupillary miotics will enable many people to function without wearing glasses. Lens-softening drops also hold promise for the treatment of presbyopia, and the results of a phase 2 trial are currently being evaluated.
1. Encore Vision announces successful phase I-II study of topical EV06 for the treatment of presbyopia [news release]. Fort Worth, TX: Encore Vision; May 5, 2016. https://www.prnewswire.com/news-releases/encore-vision-announces-successful-phase-i-ii-study-of-topical-ev06-for-the-treatment-of-presbyopia-300263690.html. Accessed February 11, 2020.
2. Garner WH, Garner MH. Protein disulfide levels and lens elasticity modulation: applications for presbyopia. Invest Ophthalmol Vis Sci. 2016;57(6):2851-2863.
3. Vargas V, Vejarano F, Alió JL. Near vision improvement with the use of a new topical compound for presbyopia correction: a prospective, consecutive interventional non-comparative clinical study. Ophthalmol Ther. 2019;8(1):31-39.



