New medical and surgical interventions have expanded the options available to patients with glaucoma. Many patients choose to begin with medical therapy instead of surgery. As with any treatment, topical glaucoma drops have side effects. A classic example is black adrenochrome deposits in the conjunctiva and cornea with topical epinephrine use. Prescribers must be aware of each agent’s common and rare side effects that may greatly affect a patient’s quality of life. This article reviews some of the side effects associated with commonly used topical antiglaucomatous agents.
ALPHA-2 ADRENERGIC AGONISTS
Alpha agonists lower IOP by decreasing the production of aqueous humor. Brimonidine is a commonly used selective alpha-2 adrenergic agonist. In 5% to 9% of patients, brimonidine can cause an acute follicular conjunctivitis 6 to 9 months after treatment initiation. A delayed follicular reaction has been reported after 15 months.1 The incidence is higher (30–48%) for apraclonidine, another alpha-2 adrenergic agonist.2
Dry mouth is a common side effect of alpha-2 adrenergic agonists, and it can be attributed to a systemic action. Children and older adults may be susceptible to central nervous system effects such as lethargy, apnea, hypotension, and bradycardia from the lipophilic nature of this drug class and its ability to cross the blood-brain barrier.3
Rarely, brimonidine causes a granulomatous uveitis that subsides upon drug cessation.4 This drug has also been reported to cause ocular lichen planus, diagnosed on conjunctival biopsy, which resolves with topical cyclosporine treatment.5
BETA BLOCKERS
Topical beta blockers also lower IOP by decreasing the production of aqueous humor. These agents are known to affect the cardiopulmonary system. Topical beta blockers such as timolol have been shown to decrease resting pulse rate, blood pressure, and spirometry values. Occurrences of severe bradycardia and complete heart block have been reported.6 Effects on the central nervous system such as confusion, fatigue, depression, and hallucinations have also been noted, especially in the elderly. Rarely, patients have reported sexual dysfunction as a side effect.7 A slight adverse effect on blood lipoproteins has also been reported with topical beta blocker use.8 Other rarely reported side effects include masking of hypoglycemia in patients with diabetes and masking of symptoms of thyrotoxicosis.
CARBONIC ANHYDRASE INHIBITORS
Like alpha agonists and beta blockers, carbonic anhydrase inhibitors (CAIs) reduce IOP by decreasing the production of aqueous humor. Oral CAIs have been associated with metabolic acidosis, hypokalemia, malaise, anorexia and weight loss, a loss of libido, depression, nausea, and gastrointestinal upset, among other effects.
Topical CAIs have largely replaced oral CAIs in glaucoma treatment, resulting in far fewer systemic side effects. The two most popular topical CAIs are dorzolamide and brinzolamide. Common side effects of topical CAIs include burning on instillation, hyperemia, blurry vision, and pruritus, all of which are likely caused by the marked acidity (pH 5.5) of the drops.9 The bitter metallic taste that some patients experience may be secondary to drainage from the tear ducts into the mouth, where carbonic anhydrase is inhibited, causing excess bicarbonate in the saliva.
Topical CAIs should be prescribed with caution in patients who have a history of corneal disease because the inhibition of endothelial carbonic anhydrase may lead to corneal edema and irreversible decompensation.10 Severe periorbital dermatitis with scaly, erythematous eyelid skin can occur 4 to 40 weeks after the initiation of dorzolamide treatment.11
A rarely reported side effect of CAIs is bone marrow suppression leading to thrombocytopenia, aplastic anemia, agranulocytosis, pancytopenia, and even death.12 Reversible thrombocytopenia has been reported with topical dorzolamide. Additionally, ciliochoroidal effusion may be induced by acetazolamide and lead to a myopic shift and angle-closure episodes.13 Finally, sulfa-related drugs such as acetazolamide have been implicated in causing toxic epidermal necrolysis (also known as Stevens-Johnson syndrome).14
PROSTAGLANDIN ANALOGUES
Prostaglandin analogues (PGAs) reduce IOP by increasing uveoscleral outflow. These agents may induce hypertrichosis, conjunctival hyperemia, irreversible darkening of the iris, darkening of the periorbital skin, prostaglandin-associated periorbitopathy (PAP), cystoid macular edema (CME), and nongranulomatous anterior uveitis.15 Hypertrichosis typically occurs 6 to 12 months after PGA initiation. Trichiasis requiring epilation has also been reported. Darkening of the iris and periorbital skin are common side effects produced from PGA-induced melanogenesis.
PAP is a series of involutional changes to the eyelids that include ptosis, lid sulcus deepening, enophthalmos, dermatochalasis, and periorbital fat loss. The condition may be particularly common with bimatoprost (> 93.3%).16 PAP can develop 1 month to 5 years after PGA therapy is initiated. The earliest finding is a loss of the periorbital fat pad.
In a retrospective review, 2.1% of patients who were administering latanoprost developed CME. The condition was associated with concurrent or past cataract surgery, either complicated or uncomplicated.17 CME is reversible with discontinuation of the PGA or the addition of a topical NSAID. One to 6 months after the initiation of latanoprost therapy, low-grade nongranulomatous anterior uveitis may be present in 4.1% to 6.4% of eyes.17 The uveitis can be resolved through the discontinuation of the PGA or the addition of a topical steroid. Rechallenging patients with a PGA may cause recurrence.
Long-term latanoprost use may worsen presbyopia via stiffening of the ciliary muscle to increase uveoscleral outflow.18 Iris cyst formation and reactivation of herpetic keratitis have also been described with PGA use.
When instilled in the eye, latanoprostene bunod ophthalmic solution (Vyzulta, Bausch + Lomb) breaks down into the active components latanoprost and nitric oxide. The nitric oxide component relaxes the trabecular meshwork and increases aqueous outflow. Latanoprostene bunod has a side effect profile similar to that of latanoprost, including hypertrichosis, conjunctival hyperemia, darkening of the iris, and darkening of the periorbital skin.19 Rarer complications have not been described to date.
RHO KINASE INHIBITORS
Rho kinase (ROCK) inhibitors cause vasodilation by smooth muscle relaxation and may induce conjunctival hyperemia and small subconjunctival hemorrhages.20 Subconjunctival hemorrhages induced by netarsudil ophthalmic solution 0.02% (Rhopressa, Aerie Pharmaceuticals), one agent in this class of medications, tend to be unilateral, small, and located adjacent to the limbus. Subconjunctival hemorrhages developed in approximately 13% of patients treated with netarsudil in phase 3 trials. Corneal verticillata occurred in 9% to 15% of patients, typically within 2 to 13 weeks of initiation of therapy with netarsudil (Figure 1). Corneal verticillata does not typically affect visual acuity and resolves about 13 weeks after drug cessation.
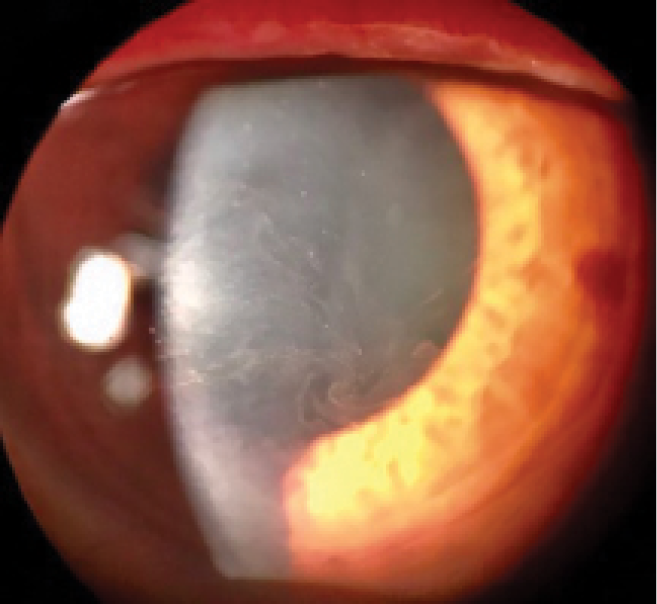
Figure 1 | Corneal verticillata caused by netarsudil.
A rarer side effect of netarsudil is reticular bullous epithelial edema, also referred to as corneal honeycombing (Figure 2). In a case series, patients developed reduced visual acuity, inferior bullous keratopathy, stromal edema, and mild anterior chamber inflammation 2 to 4 weeks after starting therapy with netarsudil.21 Significant edema was concentrated under the corneal epithelium. This side effect usually manifested in patients who had predisposing risk factors for developing corneal edema and/or inflammation (eg, history of pseudophakic bullous keratopathy, glaucoma drainage device implantation, etc.). Clinicians should therefore consider these factors before prescribing netarsudil. The edema generally resolved after drug cessation, but the visual acuity decline was permanent.
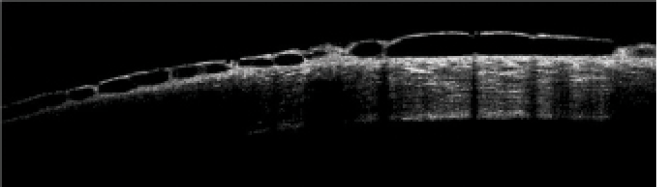
Figure 2 | OCT scan of a cornea with reticular bullous epithelial edema.
CONCLUSION
It is vital that providers be aware of the wide range of side effects associated with glaucoma medications so that they can monitor patients appropriately and halt medical therapy if issues arise.
1. Watts P, Hawksworth N. Delayed hypersensitivity to brimonidine tartrate 0.2% associated with high intraocular pressure. Eye (Lond). 2002;16(2):132-135.
2. Wilkerson M, Lewis RA, Shields MB. Follicular conjunctivitis associated with apraclonidine. Am J Ophthalmol. 1991;111:105-106.
3. Bowman RJC, Cope J, Nischal KK. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye (Lond). 2004;18(1):24-26.
4. Clemente-Tomás R, Arciniegas-Perasso CA, Hervás-Hernandis JM, García-Ibor F, Ruiz-Del Río N, Duch-Samper AM. Hypertensive acute granulomatous anterior uveitis as a side effect of topical brimonidine. Arch Soc Esp Oftalmol. 2018;93(10):511-514.
5. Ventura-Abreu N, Fernández-Aceñero MJ, Narváez-Palazón C, Romo-López A. Brimonidine-induced unilateral ocular lichen planus: a case report. Arq Bras Oftalmol. 2019;82(3):236-238.
6. Wang Z, Denys I, Chen F, et al. Complete atrioventricular block due to timolol eye drops: a case report and literature review. BMC Pharmacol Toxicol. 2019;20(1):73.
7. Fraunfelder FT, Meyer SM. Sexual dysfunction secondary to topical ophthalmic timolol. JAMA. 1985;253(21):3092-3093.
8. Coleman AL, Diehl DL, Jampel HD, Bachorik PS, Quigley HA. Topical timolol decreases plasma high-density lipoprotein cholesterol level. Arch Ophthalmol. 1990;108(9):1260-1263.
9. Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem. 2014;75:349-359.
10. Konowal A, Morrison JC, Brown SV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999;127(4):403-406.
11. Delaney YM, Salmon JF, Mossa F, Gee B, Beehne K, Powell S. Periorbital dermatitis as a side effect of topical dorzolamide. Br J Ophthalmol. 2002;86(4):378-380.
12. Fraunfelder FT, Meyer SM, Bagby GC Jr, Dreis MW. Hematologic reactions to carbonic anhydrase inhibitors. Am J Ophthalmol. 1985;100(1):79-81.
13. Man X, Costa R, Ayres BM, Moroj SE. Acetazolamide-induced bilateral ciliochoroidal effusion syndrome in plateau iris configuration. Am J Ophthalmol Case Rep. 2016;3:14-17.
14. Her Y, Kil MS, Park JH, Kim CW, Kim SS. Stevens-Johnson syndrome induced by acetazolamide. J Dermatol. 2011;38(3):272-275.
15. Camras CB, Alm A, Watson P, Stjernschantz J. Latanoprost, a prostaglandin analog, for glaucoma therapy. Efficacy and safety after 1 year of treatment in 198 patients. Latanoprost Study Groups. Ophthalmology. 1996;103(11):1916-1924.
16. Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42(2):126-131.
17. Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology. 1998;105(2):263-268.
18. Ayaki M, Tsuneyoshi Y, Yuki K, Tsubota K, Negishi K. Latanoprost could exacerbate the progression of presbyopia. PLoS One. 2019;14(1):e0211631.
19. Kawase K, Vittitow JL, Weinreb RN, Araie M; JUPITER Study Group. Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study. Adv Ther. 2016;33(9):1612-1627.
20. Serle JB, Katz LJ, McLaurin E, et al; ROCKET-1 and ROCKET-2 Study Groups. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-127.
21. Wisely CE, Liu KC, Gupta D, Carlson AN, Asrani SG, Kim T. Reticular bullous epithelial edema in corneas treated with netarsudil: a case series. Am J Ophthalmol. 2020;217:20-26.





