The Top Five
The top five research abstracts, as determined by the ME Live Program Chairs, were presented at the meeting.
Shazia Dharssi, MD
Epidemiology of Orbital Cellulitis in the United States: A 13-Year Analysis
Dharssi S, Taneja K, Rajaii F
Purpose: To determine the incidence rate, risk factors, and economic burden of orbital cellulitis in the US.
Methods: A retrospective, longitudinal study was completed using data from the US Nationwide Emergency Department Sample (NEDS) dataset. ED visits with a primary diagnosis of orbital cellulitis from 2006-2018 were included.
Results: From 2006-2018, there were an estimated 204102 ED visits in the US with a primary diagnosis of orbital cellulitis. Children (age ≤ 10) comprised a majority of cases (30.9%) with a mean age of 29.2 (95% CI, 28.5-29.8). Hypertension and tobacco use were the two most commonly associated diagnoses (p < .001) overall. When stratified by age, sinusitis and conjunctivitis were most associated with orbital cellulitis in children (p < .001). Contrary to expectation, 64.7% of cases were routinely discharged from the ED and only 27.6% of cases admitted for inpatient care. The unadjusted ED charges from 2006-2018 totaled over $353 million with a mean visit cost of $2,127.24.
Conclusion: Orbital cellulitis is a costly infection to the United States. Identifying individuals at risk for infection is vital for accurate diagnosis and triage of care.
Rodney Fong, MS, BS
Efficacy of the Treat-and-Extend Regimen in the Management of Neovascular Age-Related Macular Degeneration: 8-Year Results of the RENO Study
Fong RD, Thomas M, Romero C, et al
Purpose: The Treat-and-Extend (T&E) regimen with anti-VEGF agents is often used by retina specialists in clinical settings in the treatment of neovascular age-related macular degeneration (nAMD). This study evaluated the real-world long-term 8-year visual acuity outcomes of regular intravitreal anti-VEGF agents in patients with nAMD in the United States.
Methods: This is a retrospective, interventional, consecutive case series. 165 eyes from 137 treatment-naïve patients diagnosed with nAMD after August 2010 were treated at a single site by one physician with ranibizumab, aflibercept, bevacizumab, or brolucizumab for ≥1 year using a T&E regimen. Patients needed to receive ≥6 injections in the first year and ≥3 injections in the following years to be included in this study. Snellen best-corrected visual acuity (BCVA) was converted to ETDRS letters using a standardized formula. The main outcome measures were: BCVA change from baseline to end of patient follow-up, mean number of injections per year, and percentage of eyes losing ≥15 letters, gaining ≥15 letters or maintained vision within 15 letters.
Results: The average (standard deviation [SD]) baseline patient age was 78 years (8.5); 60% of patients were female. The mean follow-up period was 5.4 years, with 165, 158, 143, 126, 102, 78, 48, and 26 eyes completing 1, 2, 3, 4, 5, 6, 7, and 8 years of follow-up, respectively. The average BCVA at baseline was 53 letters. Mean (SD) changes from baseline in BCVA were 8.3 (21.8) letters, 7.1 (25.0) letters, 4.7 (26.5) letters, 4.5 (27.1) letters, 4.5 (28.3) letters, 5.1 (26.5) letters, and -0.5 (34.8) letters and -4.3 (38.9) letters for years 1-8 respectively. Mean number of injections during years 1-8 were 7.9, 6.1, 5.8, 6.0, 6.2, 5.9, 6.2, and 6.0, respectively. At the final follow-up, 24.2% of eyes had lost ≥15 letters, 26.1% eyes had gained ≥15 letters and 49.7% of eyes had maintained vision within 15 letters.
Conclusion: 75.8% of patients on the T&E regimen either maintained or improved their vision at the final follow-up. The T&E regimen has been effective in maintaining visual acuity in nAMD patients treated with ranibizumab, aflibercept, bevacizumab, or brolucizumab for up to six years of treatment. Years 7 and 8 had an overall average loss in EDTRS letters, however, further collection of data is needed to confirm these findings.
Harry Levine, BA, BS
Artificial Intelligence for the Identification and Quantification of Activated Dendritic Cells in Central Cornea
Levine H, Tovar A, Cohen AK, Galor A, Goldhagen B
Purpose: Activated dendritic cells (aDCs) have recently been identified as potential biomarkers indicating the presence of a systemic auto-immune disease in individuals with dry eye (DE). However, their evaluation with in-vivo confocal microscopy (IVCM) is relatively subjective. Therefore, there is a need for a standardized identification method of aDCs to improve generalizability. Our aim was to validate an algorithm that automatically identifies and quantifies aDCs using IVCM images of the central cornea.
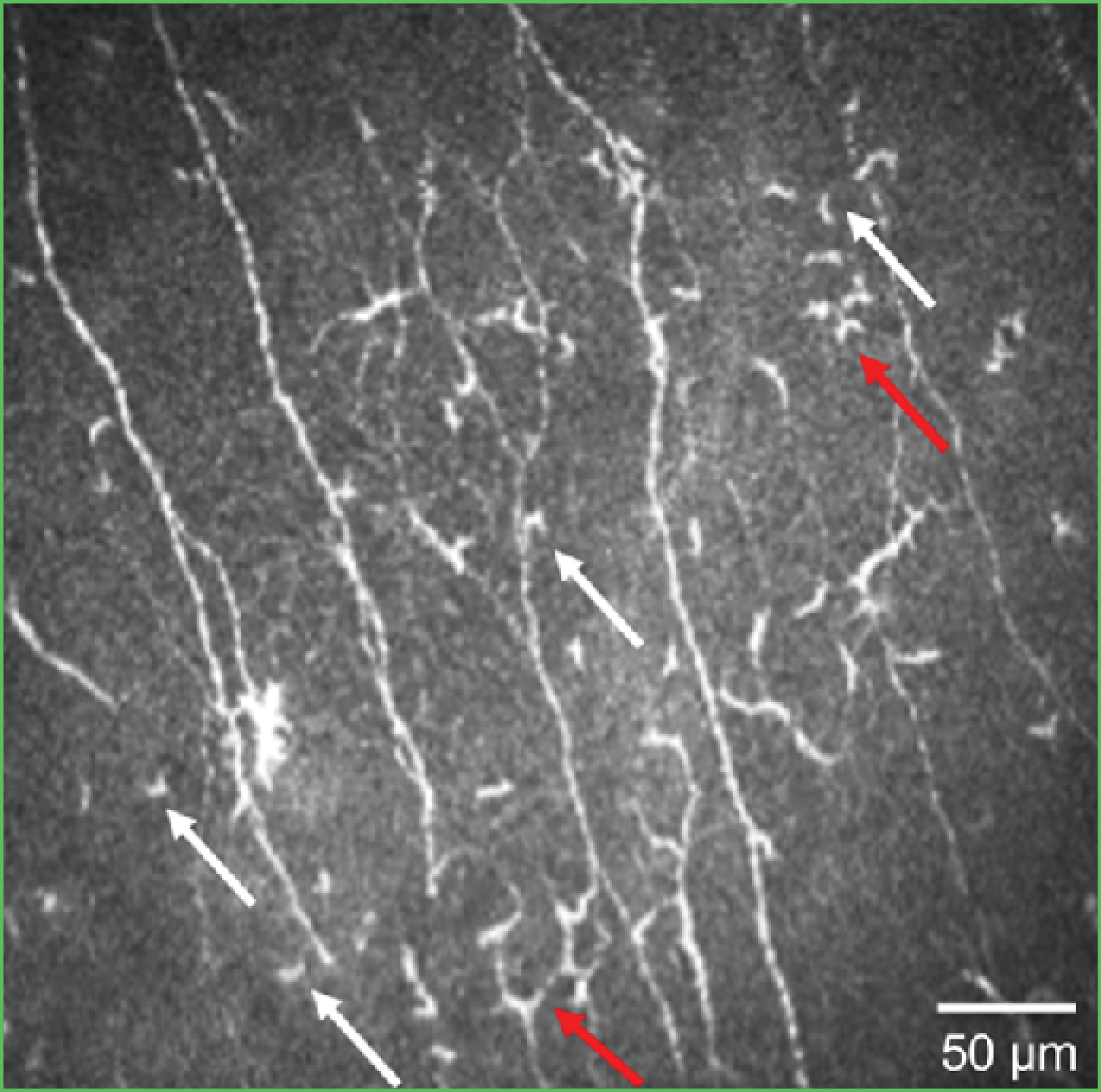
Figure 1. An IVCM image showing the evaluation of dendritic cells by morphology. Activated dendritic cells are marked in red and nonactivated dendritic cells in white.
Methods: A retrospective analysis of IVCM images obtained from individuals seen at the eye clinic at the Miami Veterans Affairs Hospital was performed. Images from individuals with corneal scarring were excluded due to potential confounding. Artificial intelligence was utilized through the use of an automated aDC counter which was developed using transfer learning with IVCM images. IVCM images used in the development of this algorithm were acquired prior to the start date of this study and did not include any of the same patients. ADCs were manually quantified based on morphology by reviewers that were masked to the algorithm findings, and intra-class correlation (ICC) was used to compare automated and manual counts. Algorithm accuracy was defined as aDC counts within 1 cell compared to manual quantification.
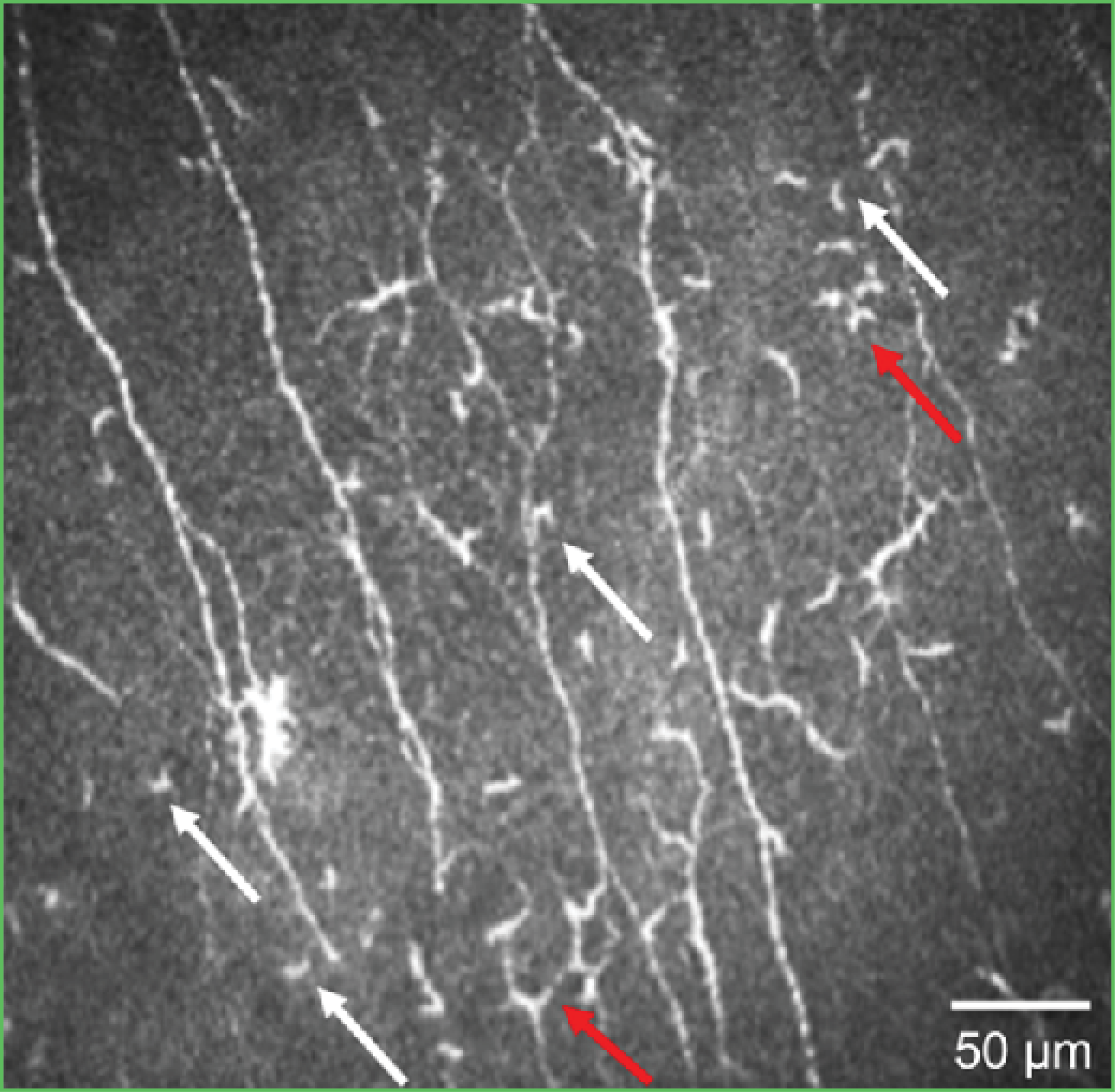
Figure 2. An IVCM image of the outcome of the AI algorithm evaluating activated dendritic cells.
Results: A total of 193 non-overlapping IVCM images from 110 individuals were included. The mean age of individuals included in the study was 55.5±18.4 years; 70.0% were male, 58.2% self-identified as White and 24.5% as Hispanic. There was no statistically significant difference in the mean aDC count between automated and manual quantifications (1.06±1.82 cells/image vs 1.21±1.98 cells/image, p-0.47). The algorithm identified a total of 207 aDCs within the dataset, out of which 184 were manually verified as aDCs. The automated algorithm performed the aDCs count with 87% accuracy and an ICC of 83% (p<.01).
Conclusion: The number of aDCs in the central cornea can be successfully estimated with the use of artificial intelligence with comparable results to manual quantification. Further assessment is needed before the widespread clinical implementation of an automated aDC counter.
Golnoush Sadat Mahmoudi Nezhad, MD, MPH
Impact of Smoking on Visual Field Progression in a Long-Term Clinical Cohort
Mahmoudinezhad G, Nishida T, Weinreb RN, et al
Purpose: To investigate the effect of smoking on the rates of progressive visual field (VF) damage over time in glaucoma.
Methods: In this longitudinal study, 354 primary open-angle glaucoma (POAG) patients with a minimum of 3 years follow-up and 5 visual field tests were enrolled from the Diagnostic Innovations in Glaucoma Study (DIGS) and the African Descent and Glaucoma Evaluation Study (ADAGES). Univariable and multivariable linear mixed models were used to investigate the effects of smoking on the rates of 24-2 VF mean deviation loss after adjusting for confounding factors such as alcohol consumption and body mass index. VF progression was defined using pointwise linear regression (three test locations having a significant regression slope (p<.01) of ≤-1dB per year). Logistic regression was used to identify whether different levels of smoking intensity were associated with VF progression. Kaplan-Meier curve and the log-rank test were used to compare the cumulative risk ratio of progression between different smoking intensity categories.
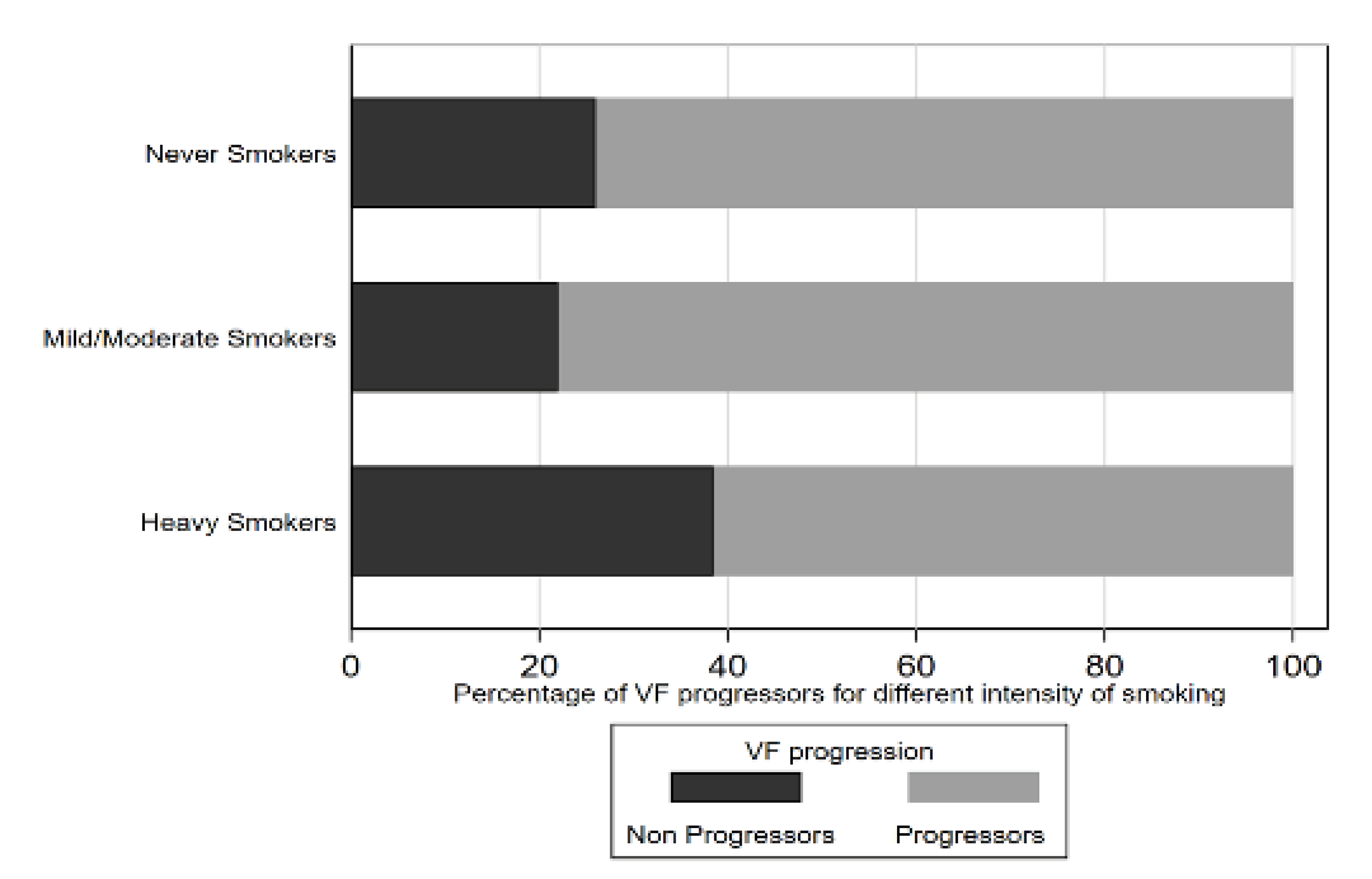
Figure 1. Graph illustrating the proportion of VF progressors for different smoking intensity categories
Results: Multicenter glaucoma registries were included over the mean follow-up of 12.4 years. Mean baseline age was 62.3 years; 124 (35%) were African ethnicity. Of the 354 patients, 168 (59.8%) and 149 (42.1%) had reported a history of smoking or alcohol consumption, respectively. 39 (11%) were heavy smokers. In a multivariable model, higher smoking intensity was associated with faster VF loss (coefficient -0.05 (-0.08, -0.01) dB/year per 10 pack-years, P=.01). Developing VF progression in eyes of heavy smokers (>20 pack-years) was 2.2 times greater than in eyes of non-smokers after adjusting for confounding factors (OR=2.21; 95% CI, 1.02,4.76; P=.04). A significantly higher proportion of progressing eyes were found in heavy smokers compared to non-smokers by Kaplan-Meier analysis (log-rank test, P<.01).
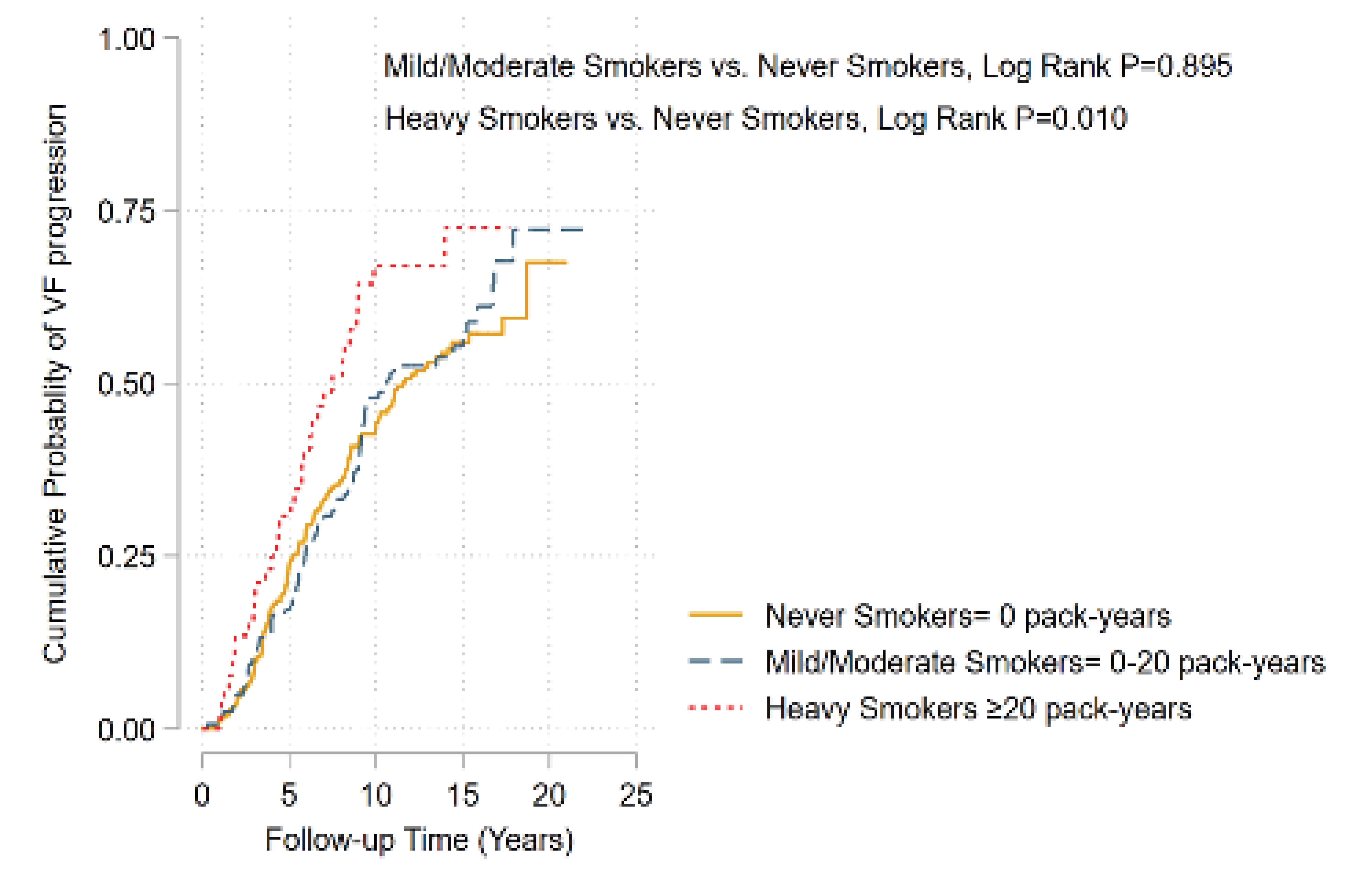
Figure 2. Kaplan-Meier analysis of the probability to detect visual field progression in glaucoma eyes.
Conclusion: Heavy smokers are more likely to have VF loss in eyes with glaucoma. The prospective longitudinal design of this study supports the hypothesis that levels of smoking may be associated with glaucoma progression. Additionally, this information can be used for clinically relevant tobacco prevention and intervention messages.
Prem Patel, BS
Electronic Communication Use in Ophthalmology: A Cross-Sectional Study Across the United States
Patel P, Hoyek S, Al-khersan H, et al
Purpose: To characterize if and how ophthalmologists communicate with patients electronically.
Methods: A survey regarding ophthalmologists’ electronic communication habits with patients was distributed online through an electronic mail (email) listserv. Univariate analysis was performed to examine associations between demographic factors and communication with patients through phone calls and email.
Results: Of 92 respondents, the average age was 55.2 years old (range: 32-86). Respondents were predominantly male (70.7%) with >10 years of attending experience (81%). Overall, 37% of respondents choose not to provide any contact information to patients, 13% provide email, 22.8% provide a phone number, and 27.2% provide both modes of contact. Reasons for sharing contact information include checking on postoperative patients (63%) or patients with complications (52.2%). Although most respondents (55.4%) do not discuss guidelines for using their personal email or phone number with patients, the majority express little or no regret sharing their contact information (87%). The majority of respondents (75.2%) endorse reimbursement for extra time talking with patients outside of clinic appointments, however only 6.4% bill their calls. Furthermore, documentation habits for communication encounters in the electronic medical record (EMR) are variable, with 44.6% of physicians documenting most of the time and 30.4% rarely documenting. Among physicians with social media accounts, most allow patients to follow their professional (57.6%), but not personal accounts (14.3%).
Conclusion: Over one-third of attending ophthalmologists do not share email address or phone numbers with patients. Ophthalmologists may share their personal information more frequently for postoperative patients and those with complications. There is widespread support for reimbursements for time spent communicating with patients outside of clinic visits, however, billing and documentation of such encounters are inconsistent.
Congratulations to All 2022 ME Live Scholarship Recipients!
- Diego Alba
- Camila Albo
- Mona Amer
- Catherine Anderson-Quinones
- Sila Bal, MD, MPH
- Samuel Barry, MD
- Eli Cehelyk
- Angela Chen, MD
- Elliot Cherkas
- Shazia Dharssi, MD
- Cherie Fathy, MD, MPH
- Rodney Fong
- Jacob D. Grodsky, MD
- Paris Hanson
- Matthew Hirabayashi, MD
- Karen Hong, MD
- Carrie Huang
- Austin Huang
- Arjan Hura, MD
- Rachel Israilevich
- Sayena Jabbehdari, MD, MPH
- Nicholas Johnson
- Gagan Kalra, MD
- Natasha Kesav, MD
- Jess Kraker, MD
- Shaina Kumar, MD
- Lauren Libfraind, MD
- Harry Levine, BA, BS
- Yalitza Lopez Pimentel, MD
- Golnoush Sadat Mahmoudi Nezhad, MD, MPH
- Zahra Markatia
- Jonathan Markle
- Harrison Marsh
- German Alberto Mejia Salgado
- Ryan Meshkin
- Zoha Mian
- Rizul Naithani
- Taj Nasser, MD
- Michael Nguyen, MD
- Prem Patel, BS
- Emily Schehlein, MD
- Gabriella Schmuter
- Ian Seddon
- Parth Shah, DO
- Sona Shah, MD
- Charlotte Shields
- Caleb Shumway, MD
- Henry Skrehot
- Brian Smith, DO
- Shanlee Stevens, MD
- Jaqueline Stoutin
- Zurriat Syed
- Joanne Thomas
- Ogul Uner, MD
- Gurpal Virdi
- Irina Volosko, DO
- Caroline Watson, MD
- Hassan Waqar
- Emily Xu
- Sanya Yadav
- Ghasem Yazdanpanah, MD, MPH, PhD
- Paul Youn
- Christopher Zhu
- Mike Zein, MD


