Since a pair of innovative therapies received FDA approval in late 2017, glaucoma specialists and their patients have had more pharmacologic treatment options than ever before. In a previous issue, we reported on one of those therapies, netarsudil ophthalmic solution 0.02% (Rhopressa, Aerie Pharmaceuticals), which is a Rho kinase inhibitor that relies on a unique mechanism of action to increase aqueous humor outflow through the trabecular meshwork.
In this issue, we look at another novel therapy that gained FDA approval: latanoprostene bunod 0.024 (Vyzulta, Bausch + Lomb). Latanoprostene bunod is a nitric oxide (NO)-donating prostaglandin F2-alpha analog that is thought to lower IOP in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) by enhancing aqueous humor outflow through the trabecular meshwork and uveoscleral outflow pathways.
UNIQUE STRUCTURE
In an interview with MillennialEYE, Paul Kaufman, MD, professor of ophthalmology and visual sciences at the University of Wisconsin-Madison School of Medicine and Public Health, described the unique structure of latanoprostene bunod. “The Vyzulta molecule has a prostaglandin backbone and a NO-donating moiety,” he explained. “These encompass two distinct mechanisms of action, one working on the uveoscleral outflow pathway—the standard prostaglandin F2-alpha mechanism—and the other working on the trabecular meshwork pathway by the standard, although not quite fully understood, NO mechanism.”
According to Dr. Kaufman, this dual mechanism of action provides a powerful effect. “When they put Vyzulta head to head against timolol in the phase 3 trials, it beat timolol at every data point. When they put it up against latanoprost, as they did in a phase 2 trial, it beat latanoprost by a little over 1 mm Hg at every time point,” he said.
CLINICAL TRIALS
FDA approval of latanoprostene bunod was based on two randomized, multicenter, double-masked, parallel-group phase 3 studies, APOLLO and LUNAR (Figures 1 and 2). These studies compared latanoprostene bunod 0.024% with timolol maleate ophthalmic solution 0.5% in patients (n = 831) with OAG or ocular OHT.1,2 The primary objective was to demonstrate that the mean IOP reduction over 3 months of treatment with latanoprostene bunod 0.024 once daily in the evening was not inferior to timolol 0.5% twice daily. A secondary objective was to demonstrate the superiority of latanoprostene bunod 0.024 once daily to timolol 0.5% twice daily. In both studies, latanoprostene bunod met the primary efficacy endpoint. The drug also showed significantly greater IOP lowering than timolol 0.5% throughout the day at 3 months of treatment, resulting in a reduction in mean diurnal IOP of 32% from baseline.
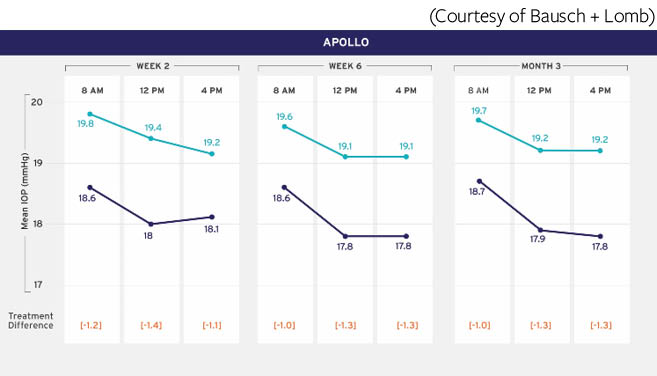
Figure 1 | In the phase 3 APOLLO study, baseline mean diurnal IOP was 26.7 mm Hg and 26.5 mm Hg in patients randomly assigned to receive latanoprostene bunod 0.024 or timolol 0.5%, respectively.
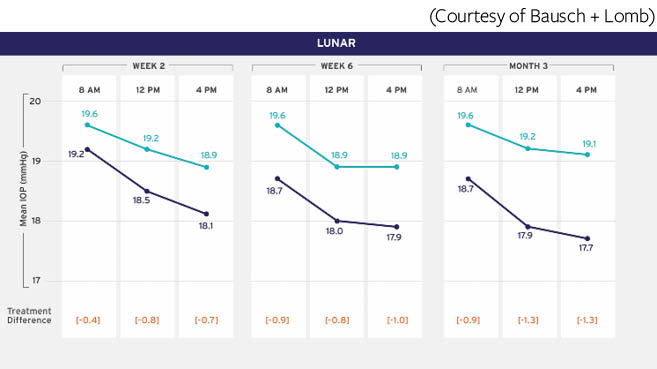
Figure 2 | In the phase 3 LUNAR study, baseline mean diurnal IOP was 26.6 mm Hg and 26.4 mm Hg in patients randomly assigned to receive latanoprostene bunod 0.024 or timolol 0.5%, respectively.
(Courtesy of Bausch + Lomb)
In a phase 2 study of 413 patients,3 treatment with latanoprostene bunod 0.024 showed greater IOP reduction than treatment with latanoprost ophthalmic solution 0.005% (Xalatan, Pfizer), with differences reaching 1.23 mm Hg (P = .005) in favor of latanoprostene bunod. More patients in the latanoprostene bunod arm achieved a mean diurnal IOP of ≤18 mm Hg compared with the latanoprost arm.
In a phase 3 study of 130 patients,4 treatment with latanoprostene bunod led to a 22% mean reduction in IOP at week 4, which was sustained for 1 year. At 1 year, the data showed a 26% reduction in IOP from baseline in the study eyes. In another phase 3 study,5 latanoprostene bunod lowered IOP over 24 hours, with a significantly greater nocturnal IOP reduction than timolol (P < .004). Compared with those treated with timolol 0.5%, patients treated with latanoprostene bunod exhibited improved daytime ocular perfusion pressure compared with baseline (P < .001) and improved nocturnal ocular perfusion pressure (P = .01) over a 24-hour period.
ROLE OF NO
In a paper reviewing the relationship between NO and the treatment of glaucoma, Aliancy et al6 concluded that latanoprostene bunod has proven to effectively, and with good tolerability, reduce IOP in patients with glaucoma and OHT. “Latanoprostene bunod capitalizes on [NO’s] ability to modulate the conventional aqueous humor outflow system, directly improving outflow through the trabecular meshwork, Schlemm canal, and distal scleral vessels,” the study authors wrote.
Dr. Kaufman explained, “The NO part of latanoprostene bunod provides an additional bang for your buck. On the basis of the clinical trials, one could expect an extra 1 mm Hg or 1.5 mm Hg of IOP lowering in virtually any patient with open-angle glaucoma. The mantra is, for every 1 mm Hg of IOP lowering, the chance of converting from OHT to manifest glaucoma decreases by about 10% over a 5-year period.7 If a patient already has glaucoma with visual field loss and glaucomatous optic neuropathy, that extra 1 mm Hg of IOP lowering decreases the risk of progression by about 10% over about a 5-year period.”8
It is too early to know how latanoprostene bunod will affect clinical treatment paradigms, according to Dr. Kaufman. However, from an adherence perspective alone it represents a positive addition, he explained. “Most of these patients are already on a prostaglandin analogue (PGA), so instead of adding another drug and expecting patients to remember to use both, by putting them on Vyzulta and discontinuing the PGA, they’re still getting the prostaglandin effect as well as another mechanism of action. The more drops we give to patients, the more difficult it becomes for them to comply and the more likely they are to either not put in their drops on an appropriate schedule or not put them in, period. With this new drug, you’re getting an extra mechanism of action without adding another drop.”
Dr. Kaufman noted that use of latanoprostene bunod has been associated with some of the ocular surface side effects expected from a prostaglandin—as well as from a rho kinase inhibitor—because these compounds are vasodilatory. “But, at least in the trials of both drugs, these ocular surface side effects did not seem any more pronounced than with standalone PGA therapy,” he said.
CONCLUSION
Dr. Kaufman predicts that netarsudil will find its true niche in the glaucoma specialist’s armamentarium once the fixed combination of netarsudil 0.02%/latanoprost 0.005% (Roclatan, Aerie Pharmaceuticals) is approved by the FDA. Dr. Kaufman remarked that NO also inhibits the Rho pathway, so it will be interesting to see how the drugs compare in practice or in clinical trials. “This will be the true test of which is the more effective drug: upstream inhibition with NO or downstream inhibition with a pure Rho kinase inhibitor, in addition to essentially the same PGA,” Dr. Kaufman concluded.
1. Weinreb RN, Sforzolini BS, Vittitow J, et al. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016;123(5):965-973.
2. Medeiros FA, Martin KR, Peace J, et al. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol. 2016;168:250-259.
3. Weinreb RN, Ong T, Scassellati SB, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738-745.
4. Kawase K, Vittitow J, Weinreb RN, Araie M. Long-term safety and efficacy of latanoprostene bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: the JUPITER study. Adv Ther. 2016;33(9):1612-1627.
5. Liu J, Slight JR, Vittitow J, et al. Efficacy of latanoprostene bunod 0.024% compared with timolol 0.5% in lowering intraocular pressure over 24 hours. Am J Ophthalmol. 2016;169:249-257.
6. Aliancy J, Stamer WD, Wirostko B. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol Ther. 2017;6(2):221-232.
7. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701-713.
8. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268-1279.



