As a glaucoma specialist practicing in 2014, I have come to value the safety of each procedure above almost everything else. If a patient is treated for glaucoma with a given therapy that has modest efficacy, the therapy can always be repeated or exchanged for an alternative treatment—as long as it is safe and does not harm the patient. In most patients who experience adverse events, for example loss of visual acuity from trabeculectomy, the efficacy of the procedure will typically be viewed as a sour consolation prize, even if, in fact, the treatment was in the patient’s best interest. Side effects tend to be cumulative, and in a disease that could require many courses of therapy, an accumulation of side effects is suboptimal. Of course, each patient scenario is unique. In some patients with advanced disease on presentation, or for whom follow-up is questionable, it may be safer to perform a highly effective, definitive procedure even if there is a sizable risk of adverse events, as the patient may have time to undergo sequential stepwise escalations of therapy.
In a study that I found to be quite enlightening, Douglas Rhee, MD, and colleagues compared ab interno trabeculectomy, also known as the Trabectome (Neomedix) technique, to standard trabeculectomy.1 The percentage of patients whose pressure was under control 2 years after trabeculectomy was 76%, while only 22% were controlled with the Trabectome. However, complications occurred in only about 4% of the Trabectome group but in 35% of those receiving standard trabeculectomy. When you consider that half of all trabeculectomies fail after 5 years, combining a series of trabeculectomies over a patient’s lifetime, each with a 35% complication risk, becomes problematic. Of course, the Trabectome is not really repeatable, so alternative therapies would be needed.
For this reason, I have attempted to focus my glaucoma practice on the safest procedures, which includes microinvasive glaucoma surgery (MIGS). In moderate glaucoma, when possible, I combine the iStent (Glaukos), cataract extraction, and endocyclophotocoagulation (ICE). Robert Noecker, MD, MBA; Steve Sarkisian Jr, MD; Parag Parekh, MD; and I reviewed our initial data of 48 patients and found that the ICE procedure does lower IOP in a moderate manner and has an exceptional safety profile. After 3 to 6 months, 47% of our patients had achieved a 20% pressure reduction.
One treatment that traditionally has not been thought of as a MIGS procedure is transscleral cyclophotocoagulation (TSCPC), performed with an 810-nm laser and the use of the G-Probe (Iridex). The downside to the procedure, which can lower intraocular pressure (IOP) by up to 50%, is that it has been associated with inflammation, macular edema, and (rarely) phthisis bulbi; however, TSCPC has mostly been studied in populations with serious eye pathology, such as neovascular glaucoma. Yet, there are many aspects of TSCPC that would make it an ideal MIGS procedure. TSCPC can be performed in essentially any eye, with any type of glaucoma mechanism (particularly angle-closure glaucoma), and before or after any other glaucoma procedure. It is repeatable and titratable, and it does not require implanted hardware, use toxic anti-metabolites, have any risk of infection, or create a filtering bleb. TSCPC can also be performed with a mobile unit about the size of a briefcase in semisterile conditions. The main limitation of TSCPC from a practical standpoint is that a retrobulbar block is usually required, although these can be given in an office or surgery center setting.
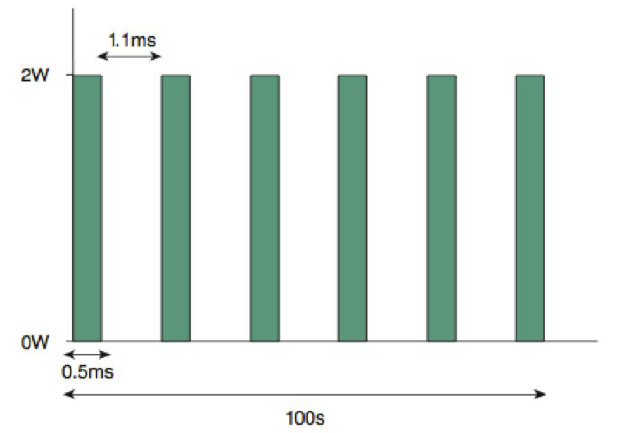
With the TSCPC micropulse technique, the laser is applied for 50 seconds, alternating between 0.5 ms of treatment "packets" and 1.1 ms of rest.
So, how could the TSCPC procedure develop a better safety profile? The first idea is to perform the “slow burn” technique, pioneered by Doug Gaasterland, MD. With this technique, a longer duration of laser energy is delivered with lower powers, with the goal of avoiding vaporization of the ciliary processes. So, for example, instead of delivering 2,000 mW for 2 seconds, one might deliver 1,250 mW for 4 seconds. This approach reduces tissue destruction and pain and results in less inflammation. Recently, a group of physicians have been investigating a new micropulse glaucoma device developed by Iridex. Iridex’s proprietary MicroPulse technology has been gaining broader acceptance in both the treatment of retinal conditions and trabeculoplasty, as it has demonstrated its ability to stimulate a therapeutic response without burning tissue. This new laser will incorporate the MicroPulse technology into an infrared platform to support transscleral procedures. In this 2- to 3-minute, nonincisional procedure, micropulse laser energy is delivered with a specially designed probe. The mechanics of MicroPulse are similar to a pulse mode using phacoemulsification or (in my mind) to antilock braking systems. By allowing brief pulses of rest during the application of the laser energy, one can inhibit thermal spread and allow better targeting of laser energy with less thermal collateral damage. The published 18-month data2 by Paul Chew, MD, demonstrates substantial IOP reduction, and as the study authors indicate, “The rapid reduction in IOP seen may be due to enhanced uveoscleral outflow.” Further study is required, but my initial experience with this new technology has demonstrated IOP reduction with minimal postoperative inflammation.
In summary, glaucoma care is evolving and putting safety first. Repeatable, titratable procedures with favorable safety profiles can provide scalable options for the long-term control of glaucoma.
1. Jea SY, Francis BA, Vakili G, Filippopoulos T, Rhee DJ. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology. 2012;119(1):36-42.
2. Aquino MCD, Tan AM, Loon SC, See J, Chew PT. A randomized comparative study of the safety and efficacy of conventional versus micropulse diode laser transscleral cyclophotocoagulation in refractory glaucoma. Paper presented at: the 2011 Association for Research in Vision and Ophthalmology meeting; May 3, 2011; Ft. Lauderdale, FL.



