Glaucoma is one of the leading causes of blindness around the world. Today, approximately 60 million people worldwide are affected by the condition, and that number is projected to increase to more than 80 million by 2020.1 Annually, $6 billion is spent worldwide in the glaucoma treatment market. Clearly, this is a large and significant space in regard to the number of patients affected by glaucoma and the amount of money invested in its prevention and treatment.
Current glaucoma therapy has significant limitations. Due to compliance issues, poor persistence, side effect profile, and lack of efficacy, medical therapy has failed many patients. Laser trabeculoplasty has shown safety, but there are issues with response rate and attrition. We are also all well aware of the complications associated with trabeculectomy and tube shunt surgeries, including loss of vision.2,3 Therefore, traditional glaucoma surgery is typically used as a last resort for refractory cases, usually in patients with advanced disease.
AN UNMET NEED
In an average practice, about 10% of glaucoma patients have advanced disease, requiring surgery, and 30% have mild disease and do well on one or two medications. However, 60% of patients remain in that middle class, which is often the neglected class within a patient population. These are individuals who are taking multiple medications, whose IOP is not ideally controlled, and who could truly benefit from a new treatment option—this is the need that microinvasive glaucoma surgery (MIGS) attempts to address. A gap exists between medication and lasers, and trabeculectomy and tubes, and we aim to change the way we treat glaucoma by filling this gap with MIGS and drug delivery.
It is important to differentiate the MIGS patient from the trab patient. Trab patients have advanced disease, progressive normotensive glaucoma, and lower IOP targets of less than 12 mm Hg. In contrast, MIGS patients typically have mild to moderate disease, hypertensive glaucoma, and more modest IOP targets of 15 to 16 mm Hg.
Glaucoma is a source of significant optic neuropathy and a significant cause of blindness in the world, as stated above. Like some of my colleagues, I have always felt that elevated IOP is truly a surgical problem; it is not a medical problem. When considering glaucoma and glaucoma treatment, MIGS can be thought of as potentially glaucoma surgery for the masses—for the average ophthalmologist and for the average patient. It potentially answers the need for a straightforward glaucoma treatment that can be used in all operating rooms around the world.
Change will be a bit difficult in the glaucoma space. Some innovation is sustained, some innovation is evolutionary, and some innovation is revolutionary. MIGS is truly an area that is disruptive: It is creating a new interventional space within glaucoma treatment, targeting patients and surgeons who are not best served by current treatment modalities.
DEFINING MIGS
MIGS is defined as a microinvasive ab interno approach. Now, microinvasive does not refer to incision size; instead, it means a reduction in the manipulation of tissue and the retention of normal anatomy and physiology in the best possible way. That is truly what MIGS technology is about and why, above all, it is an extremely safe approach. MIGS outcome measures include not only the lowering of IOP but also the reduction and elimination of medications—that is a potentially confounding issue, but for our patients, it is a very important outcome in terms of how well these treatments work.
COMBINED CATARACT AND GLAUCOMA SURGERY
We have clearly seen a merger of cataract and refractive surgery in the past 10 or 15 years. We are now seeing a merger of cataract surgery and glaucoma, where phaco is used as a platform to add synergy with MIGS devices to provide further IOP lowering beyond what phaco can do alone. After all, many of our patients who undergo cataract surgery (15% to 20% in the United States) have comorbid glaucoma that could be well suited to treatment with a combined procedure.
MIGS PROCEDURES AND PATHWAYS
It is best to classify the various MIGS procedures by their outflow targets, which include: (1) Schlemm canal, (2) the suprachoroidal space, and (3) the scleral/subconjunctival space.
Schlemm Canal
Schlemm canal is the conventional outflow pathway for which various microstents are being investigated. Common devices using this pathway include the iStent (Glaukos), iStent Inject (Glaukos), Hydrus (Ivantis), and Trabectome (NeoMedix Corporation).
The iStent is the most commonly used microstent, particularly in the United States. It is the smallest medical device, measuring 1 mm in size, and is designed to bypass the trabecular meshwork. Early studies examined the use of a single iStent; however, many of us have now moved on to using multiple iStents. Basic science and emerging clinical data indicate that multiple bypasses allow for a greater reduction of pressure by reducing outflow resistance over a larger capacity of outflow vessels. As solo procedures (ie, not combined with phaco), IOP reduction has been found with microstents, indicating that these devices do work on their own. Further innovation will likely occur in this space with the use of multiple targeted iStents, injectable microstents, and scaffolding microstents.
As canal surgeons, our goal is to basically reestablish flow to the episcleral venous plexus, but not every vein is equal. Our goal for placing microstents is to target the aqueous veins emerging directly from the canal. There are only four to six of these veins in any given eye. We address these veins by looking at the episcleral surface and at focal blood reflux, as blood reflux implies that an aqueous vein is present right behind the canal. To identify aqueous veins, we like to mark these positions like we would mark a toric IOL so that these devices are placed in the accurate position, as opposed to random placement. Not every point in the canal will drain the same amount as another point identified by a target. So, we identify these points and place the stent(s) over the areas where blood reflux is present. Microstent implantation, while elegant, is technically demanding. Although we like to think of MIGS procedures as being safer and yielding smoother recovery, surgical acumen is a requirement for their success.
There are alternative ways to approach Schlemm canal. The Hydrus Microstent is a scaffolding device that doesn’t just bypass the trabecular meshwork but spans 3 clock hours to allow a free flow of aqueous directly into the canal.
Suprachoroidal Space
The suprachoroidal space is the nonconventional outflow pathway with vast resorptive potential and the ability to lower IOP substantially. Data have shown that there is a significant pressure gradient from the anterior chamber to the suprachoroidal space and that this gradient increases with increasing IOP and the distance from the limbus. Both the Cypass Micro-Stent (Transcend Medical) and the iStent Supra (Glaukos) devices are designed to shunt aqueous into the suprachoroidal space.
Scleral/Subconjunctival Space
The scleral/subconjunctival space is a very familiar pathway in traditional glaucoma surgery. This pathway is targeted by the Xen Gel Stent (AqueSys), a 6-mm flexible, soft, gelatin device designed to shunt aqueous from the anterior chamber to the subconjunctival space. The Xen stent is available in three sizes with different outflow capacities, and appears to be the more potent of the current MIGS devices, albeit with some slightly increased risk—hence the term MIGS Plus seems appropriate for this approach.
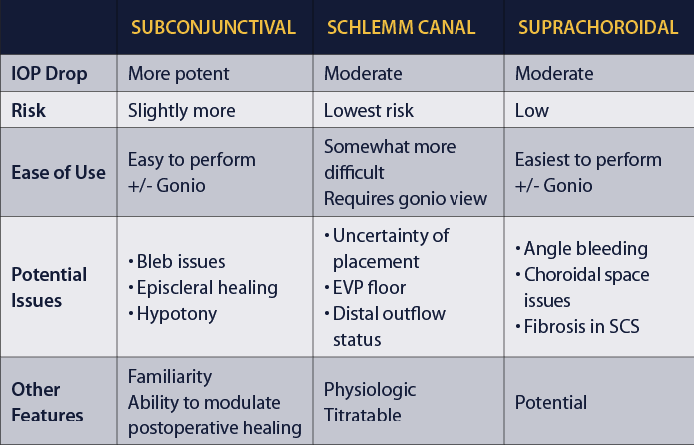
RISK, REWARD, EFFORT
The table below features a comparison of MIGS outflow pathways and devices.
Some MIGS procedures are easier to perform, while others are a bit more challenging. Each has its own potential issues to be aware of, whether related to the device or to the areas around where the device is placed. As surgeons, we can choose from these devices based on the risk-reward-effort ratio. This is a matter of deciding the risk of the procedure versus the risk of the disease and how far we are away from the target. Like other glaucoma therapies, each MIGS procedure carries its own safety and efficacy profile, and more data on these outcomes are forthcoming.
THE FUTURE OF MIGS
The promise of MIGS is to intervene early in disease using synergy with phaco if necessary to reduce morbidity of progression. This approach is predicated on greatly enhanced safety and will hopefully reduce the need for more aggressive surgical therapy later in disease. Any glaucoma patient proceeding to phaco should be considered for a combined cataract-MIGS procedure. In the future, MIGS procedures will likely be used as solo procedures more and more in phakic and pseudophakic eyes. We will also see MIGS followed by MIGS as well as drug delivery, which will be an important innovation in the next few years.
There are a variety of factors that we still need to consider for MIGS, including:
• Long-term IOP-lowering data
• Patient selection and appropriate outflow target
• Technical ability
• Distal outflow disease
• Patency/lumen obstruction
• Fibrosis/encapsulation
• Cost, reimbursement, and regulatory challenge
• Competition with drug delivery
If we look at our current glaucoma treatment algorithm, perhaps we will see a change in 5 years, where drug delivery is used earlier in disease and MIGS is used earlier with or without combination phaco. This is a very exciting time not only for glaucoma surgeons and patients but for all surgeons in the ophthalmic space.
1. National Eye Institute. Facts About Glaucoma, Glaucoma Symptoms. Available at: https://www.nei.nih.gov/health/glaucoma/glaucoma_facts.asp#a. Accessed October 1, 2014.
2. Gedde SJ; for the Tube Versus Trabeculectomy Study Group. Results from the Tube Versus Trabeculectomy study. Middle East Afr J Ophthalmol. 2009;16(3):107-111.
3. Lichter PR, Musch DC, Gillespie BW, et al; for the CIGTS Study Group. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or sugery. Ophthalmology. 2001;108(11):1943-1953.



