
Brandon D. Ayres, MD
Game Changer… Life Changer
When I was asked to comment on a game changer, my mind first went to new fancy technology like femtosecond lasers, three-dimensional imaging, intraoperative aberrometry, and the like. The more I thought about it, the more it appeared to me that these technologies may have advanced cataract surgery … but it is still cataract surgery, just better. These may be more of a game advancer, not so much a game changer. In my opinion, the single most game-changing device is the iris prosthesis.
Iris repair and replacement has been a passion of mine for as long as I can remember having an interest in ophthalmology. Iris repair tends to be a tedious surgery with mixed results. Some pupils may end up too big, too small, or misshapen. If enough of the iris has been damaged or lost, repair may not be an option at all. In cases where there is not enough iris to repair, replacement is a better option. The problem with replacement is that there is no FDA-approved iris prosthesis to date. With a lot of paperwork to gain FDA and IRB approval, compassionate use of a variety of devices may be possible, but it can be a long process and very frustrating. Many of the prosthetic devices have a limited color range, making it difficult to match the contralateral iris, or the colors may look very artificial in ambient light.
Over the past year, I have had the privilege of being a part of the Human Optics Artificial Iris-001 (HO AI-001) FDA trial. In this trial, we are examining the effectiveness and safety of a silicone color-matched iris prosthesis for partial and complete aniridia. The device is a silicone iris that has the look and feel of an actual iris. Pictures of the unaffected iris (sometimes there is not one in aniridia) are taken, and painstaking efforts are made to try and match the color and texture. The implant can be placed in the capsular bag or sulcus or sutured to the scleral wall. Having the ability to use this iris prosthesis is definitely a game changer for me.
Not only is the artificial iris a game changer for the surgery, it is a game changer for the patient, in some cases even a life changer. It is not often you see patients shed tears of joy after surgery. The tears are not because of their improvement in vision; it’s because they feel whole again. They’re finally not feeling self-conscious or worried about people asking questions. They finally have what many doctors told them they could never have: a new iris and a new outlook on life.
The HumanOptics CustomFlex Artificial Iris device is investigational and not yet approved by the FDA.
Brandon D. Ayres, MD, is a surgeon in the Cornea Service at Wills Eye Hospital in Philadelphia. Dr. Ayres was a clinical investigator in the HO AI-001 FDA trial. He may be reached at (484) 434-2700; bayres@willseye.org.
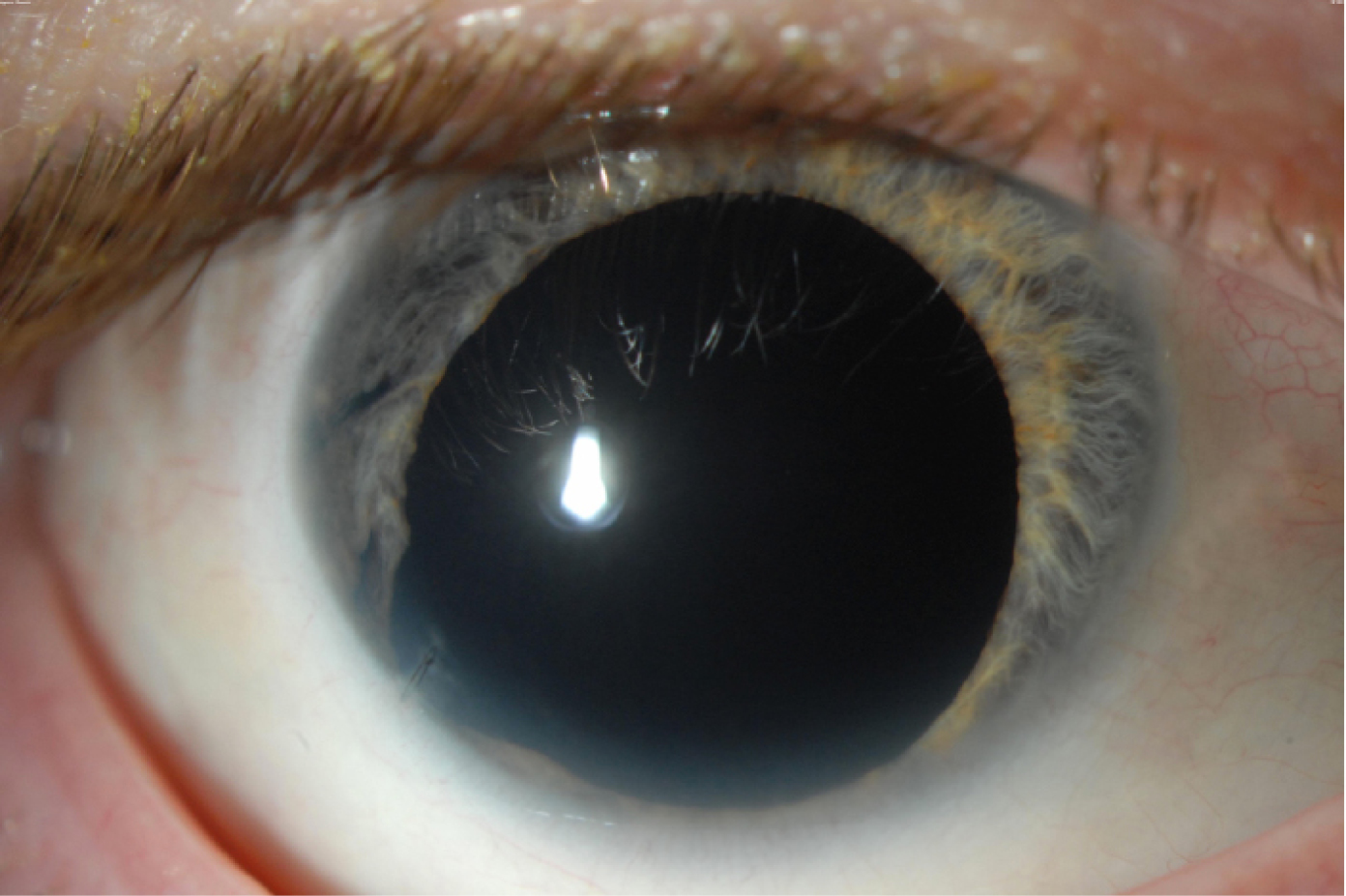
A patient with aniridia and aphakia prior to surgery.
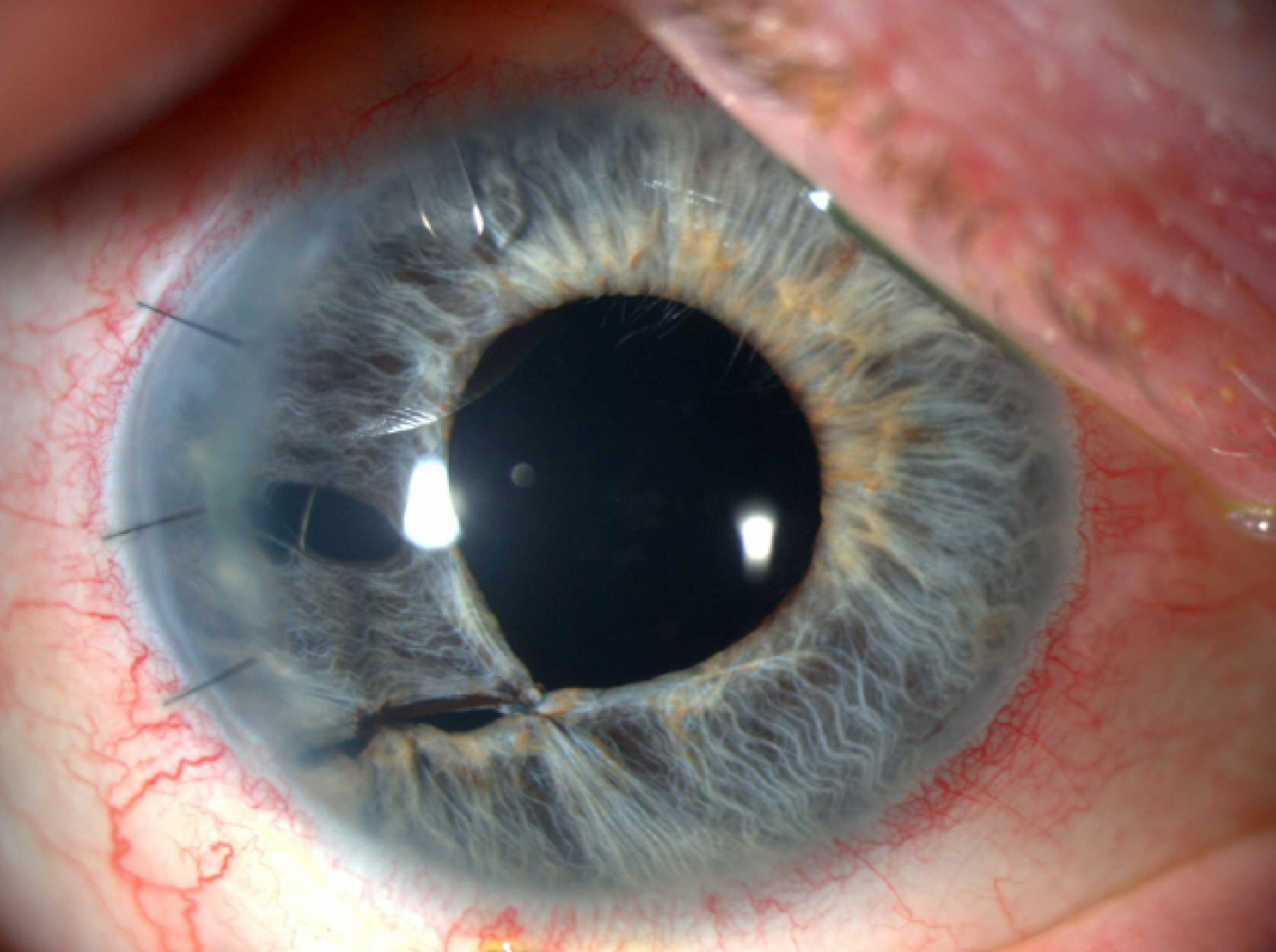
A patient after surgical repair of iris and secondary sutured IOL placement.

Morcher iris prosthesis color match system. PMMA IOLs are compared with the patient’s iris color. Once the correct color is selected, the IOL can be ordered with the correct dioptric power. This is a large (10 mm) nonfoldable IOL.
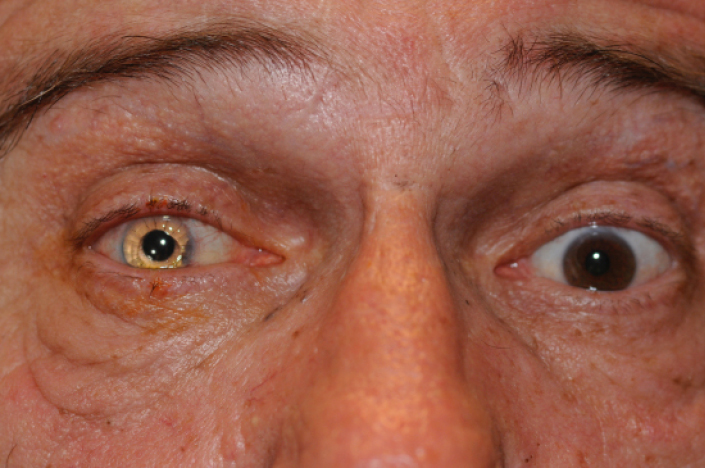
A postoperative photo of a patient with a Morcher color-matched IOL. The right eye has a reflector-like quality, and the color match is poor.

Dr. Schmidt Human Optic silicone iris prosthesis. The silicone iris has not only the color but also the texture of a natural iris.
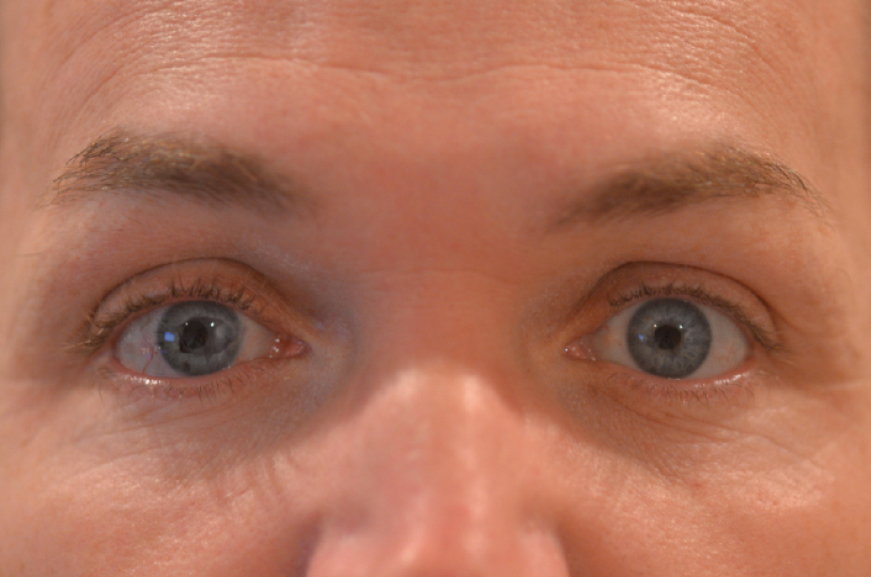
A postop patient about 3 months after secondary IOL and iris prosthesis surgery on the right eye.
Traumatic cataract and iridodialysis repair.
Traumatic cataract with artificial iris implant.

Kathryn M. Hatch, MD
Intraoperative Aberrometry
The ORA system (Alcon) has been a game changer in my practice. As an intraoperative aberrometer, this device gives real-time aphakic and pseudophakic refractions as well as keratometry (K) information for limbal relaxing incision (LRI) management. The aphakic refraction provides both a spherical and toric power calculation for optimal IOL power selection to achieve the refractive target. In addition to aphakic power calculation, the pseudophakic refraction, specifically in cases of toric IOL implantation, allows for optimal axis alignment. After IOL implantation, a pseudophakic refraction is obtained and one of the following rotation recommendations is provided: clockwise, counterclockwise, or NRR (no rotation required). If clockwise or counterclockwise is encountered, the surgeon can opt to reposition the IOL.
In addition, LRI management can be done and titrated based intraoperative K readings. A reading is obtained prior to manual (or after femtosecond laser-created) LRIs, and the K reading and axis is provided. The surgeon can then titrate the LRIs based on the readings. This function can be performed and managed in phakic, aphakic, and pseudophakic states. The speed and accuracy of the technology was improved with the VerifEye hardware upgrade, released in July 2013. With this upgrade, eye stability is confirmed and real-time refractions are viewed in the preview screen.1
The ORA has proven to be a game changer for my practice, as I now routinely use this technology for all eyes in which I am planning a specific refractive cataract surgery situation, including toric IOL implantation, post-laser vision correction, post-RK eyes, eyes with long or short axial lengths, or cataracts for which we cannot obtain accurate biometry. I also am using the ORA to optimize my manual or femtosecond LRIs. We may be learning that with refractive cataract surgery, you don’t always “get it right the first time.” In our practice, we have found that a change to the toric IOL power was made 25% of the time and that at least one IOL rotation was required in one-third of cases with ORA. We also found that we could reduce postoperative residual refractive astigmatism and were approximately two-and-a-half times more likely to achieve ≤0.50 D residual refractive astigmatism with the use of the ORA compared with standard techniques.
The ORA may be shown to reduce the need for postoperative laser vision correction enhancements, and it may change the way we approach refractive cataract surgery—a true game changer.
Kathryn M. Hatch, MD works at the Massachusetts Eye and Ear Infirmary, Waltham, and specializes in cornea, cataract, and refractive surgery. She is a member of the Faculty in Ophthalmology at Harvard Medical School. Dr. Hatch states that she has no financial interest in the products or companies mentioned. She may be reached at Kathryn_hatch@meei.harvard.edu
1. WaveTec Vision. WaveTec Vision Announces the Commercial Launch of VerifEye for the ORA System [news release]. July 9, 2013. Available at https://wavetecvision.com/wavetec-vision-announces-the-commercial-launch-of-verifeye-for-the-ora-system/. Accessed October 10, 2014.

The Ora intraoperative aberrometer gives real-time aphakic and pseudophakic refractions as well as keratometry information for LRI management.

William F. Wiley, MD
LAL: It’s No Laughing Matter!
I foresee the Light Adjustable Lens (LAL) from Calhoun Vision as one of the next game changers in ophthalmology. Since the advent of corneal refractive surgery in the 1990s, providers and patients have become obsessed with surgical refractive vision correction. Cataract surgery then soon followed suit, and we have seen progressive advances in technology allowing us to push the limits of obtaining successful refractive outcomes. I see the LAL as the next major step forward in achieving these refractive visual outcomes.
Our personal trial results have exceeded outcomes I have seen in either LASIK or cataract surgery. Our early results have shown that 100% of our patients are seeing 20/20 or better, and 56% 20/16 or better. We are at a point where there is a slight level of disappointment felt by the staff when patients are “only” seeing 20/20. Furthermore, 100% of the patients have spherical equivalent of 0.25 D or less.
The postrefractive cataract surgery market continues to grow as our initial LASIK/RK patients age, and I see the LAL as the future gold standard for achieving the desired refractive outcome. Technology is being developed to help not only achieve distance correction with the LAL but also tackle the presbyopia problem with extended depth of focus treatment patterns. I can foresee demonstrating the treatments with trial lenses or contacts prior to lens adjustment to help educate our patients and ensure that they are receiving the desired effect. This begins a new era of needed customized outcomes just as patient expectations are beginning to peak outside of what our current technology can deliver. n
William F. Wiley, MD, is the Medical Director of the Cleveland Eye Clinic and an Assistant Clinical Professor of Ophthalmology at University Hospitals/Case Western Reserve University in Cleveland. Dr. Wiley states that he has received research funding from Calhoun Vision. He may be reached at (440) 526-1974; drwiley@clevelandeyeclinic.com.

The Light Adjustable Lens.

Marcony R. Santhiago, MD, PhD
PTA: Percent Tissue Altered
There is an integrated relationship between preoperative corneal thickness, ablation depth, and flap thickness in determining the relative amount of biomechanical change that has occurred after a LASIK procedure. We have proposed and investigated a new metric, the percent of anterior tissue depth altered, or percent tissue altered (PTA), that describes this interaction during excimer laser refractive surgery, which for LASIK can be described as: PTA = (FT + AD)/CCT, where PTA = percent tissue altered, FT = flap thickness, AD = ablation depth, and CCT = preoperative central corneal thickness.
While most patients who have developed ectasia after LASIK have, in retrospect, had identifiable risk factors, particularly irregular topographic patterns, that placed them at higher risk for this complication, ectasia cases in patients with normal preoperative topography still present a conundrum. Our studies provided evidence that a high value of PTA, especially greater than 40%, is a relevant factor in the development of post-LASIK ectasia in eyes with normal preoperative Placido disc-based topography, and, therefore, PTA should be taken into account as a screening parameter for refractive surgery candidates.1-3 This metric more accurately represents the risk of ectasia than the individual components that comprise it.
Because the cohesive tensile strength is not uniform throughout the central corneal stroma and the anterior 40% of the corneal stroma has significantly greater cohesive tensile strength, removing this relevant part of the stroma may induce corneal weakening in increasing proportion as the threshold of 40% is reached and crossed. Compared with specific residual stromal bed (RSB) or CCT values, PTA likely provides a more individualized measure of biomechanical alteration because it considers the relationship between thickness, tissue altered through ablation and flap creation, and ultimate RSB thickness.
Compared with other variables, PTA had higher prevalence, higher odds ratio, and higher predictive capabilities for ectasia risk than moderate to high Ectasia Risk Score System values, RSB, CCT, high myopia, ablation depth, and age. PTA ≥40 was a more robust indicator of risk than other variables in patients with normal preoperative topography, being even more sensitive than the absolute cutoff value of the RSB (300 μm) itself that influenced the risk of ectasia the most.
Marcony R. Santhiago, MD, PhD, is Head of the Cataract Surgery Department and a Professor of Ophthalmology at Federal University of Rio de Janeiro as well as an Associate Professor at the University of Sao Paulo in Brazil. Dr. Santhiago states that he has no financial interest in the products or companies mentioned. He may be reached at email: marconysanthiago@hotmail.com.
1. Santhiago MR, Smadja D, Wilson SE, et al. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014;158(1):87-95
2. Santhiago MR, Kara-Junior N, Waring GO IV. Microkeratome versus femtosecond flaps: accuracy and complications. Curr Opin Ophthalmol. 2014;25(4):270-274.
3. Santhiago MR, Wilson SE, Hallahan KM, et al. Changes in custom biomechanical variables after femtosecond laser in situ keratomileusis and photorefractive keratectomy for myopia. J Cataract Refract Surg. 2014;40(6):918-928.

Robert J. Weinstock, MD
Game-Changing Ophthalmic Surgical Visualization
It’s clear that digital imaging is one of the greatest innovations of our time. Digital cameras (now in our phones) are changing everything. Images can be edited, enhanced, and transmitted in ways not possible with optical and/or film predecessors. Endoscopic surgery, robotic surgery, medical diagnostics, and even Facebook could not exist without digital imaging.
TrueVision is adding a surgical chapter to the digital imaging book of inevitability.
The standard of care for ophthalmic surgical visualization is the optical stereomicroscope. This is one of the last bastions of nondigital imaging in medicine today. TrueVision is positioned to replace current optical microscopes with a digital platform within 3 to 5 years. After 6 years of development and refinement, the market appears to be at a tipping point with TrueVision’s intelligent digital visualization platform. TrueVision has recently reached parity with the optical image quality of the microscope with their fifth-generation system.
In addition, TrueVision enables information to be added to the surgical process in ways that are currently impossible. Accordingly, TrueVision can dramatically improve the surgical process for both surgeons and patients. TrueVision has created its novel three-dimensional (3-D) visualization platform and is now adding software applications that deliver computer guidance for specific surgical procedures. The combination of digital 3-D visualization and computer guidance enables the surgeon to see better and become more precise and efficient. TrueVision is a digital visualization platform that offers benefits across all subspecialties including anterior segment, posterior segment, glaucoma, and LASIK.
In early studies with TrueVision’s first guidance application for astigmatic correction (TrueGuide), patients achieved correction to 0.50 D or less, 83% using toric IOLs (Solomon) and 86% using limbal relaxing incisions (Weinstock, Packer, and Katsev).
With TrueVision, you can now tailor a digital surgical cockpit (similar to a fighter pilot) to an individual surgeon or practice-specific configuration. The cockpit will present to the surgeon all relevant patient information, including diagnostic data, completely integrated with a live 3-D view. This allows surgeons to make better decisions in real time with maximum efficiency and accuracy and avoid ergonomic issues that have plagued the optical microscope.
Robert J. Weinstock, MD, is a cataract and refractive surgeon in practice at The Eye Institute of West Florida in Largo, Florida. Dr. Weinstock states that he is a consultant to TrueVision. He may be reached at (727) 585-6644; rjweinstock@yahoo.com.

Kendall E. Donaldson, MD, MS
Astigmatism Correction With Laser Cataract Surgery
No matter what your opinion on femtosecond laser cataract surgery might be, I think we would all agree that the introduction of lasers into cataract surgery has changed the focus of surgeons. Laser cataract surgery is as controversial as ever since its introduction into mainstream cataract surgery with FDA approval in 2011. Some surgeons insist that their results are better with the laser, and others insist traditional cataract surgery was already optimized without the use of a laser. However, for those of us who have embraced this new technology, we seem to have heightened the focus on astigmatism correction to a whole new level. Personally, prior to using the laser, I performed manual limbal relaxing incisions (LRIs) infrequently. I found my results to be somewhat unpredictable and often achieved minimal astigmatism correction with peripheral relaxing incisions, despite taking courses with astigmatism wizards like Skip Nichamin, MD, and Eric Donnenfeld, MD. When moving my incisions more central to achieve a greater effect, I had the occasional unfortunate experience of inducing irregular astigmatism.
We’ve learned that more than half of our patients presenting for cataract surgery have at least 0.75 D of preexisting corneal astigmatism, according to Dr. Warren Hill’s Keratometry Database.1-2 Although these patients are potential candidates for astigmatism correction, the majority are still receiving only spherical correction at the time of their cataract surgery. I see this as a missed opportunity for increased precision and improved outcomes. Over the past decade, we have seen the evolution of cataract surgery from simply removal of a cloudy lens to a refractive vision correction procedure. During this time of technological evolution, we have also experienced a parallel increase in patient expectations. Fortunately, the two have risen at a similar rate, so we are able to provide the best outcomes if we use the technology available to us.
Now with laser cataract surgery, I perform LRIs in almost every case, and when using the femtosecond laser with a toric IOL, I use intrastromal marking incisions, which help ensure accurate alignment of the IOL (along with the use of the ORA intraoperative aberrometer [Alcon]). I feel very comfortable with the placement and accuracy of my incisions using the laser. I particularly look forward to using Verion (Alcon) and other intraoperative imaging systems that help optimize our outcomes using postoperative data to continuously develop and optimize each surgeon’s customized nomograom.
Whether you are an advocate of laser cataract surgery or not, it’s undeniable: The increased attention being paid to astigmatism correction and surgeon-specific nomogram creation has been a great “side effect.” It’s taken us one step closer toward the ultimate quest for emmetropia. Watch out, LASIK!
Kendall E. Donaldson, MD, MS, is an Associate Professor of Clinical Ophthalmology at Bascom Palmer Eye Institute in Miami and the Medical Director of Bascom Palmer Eye Institute at Plantation. Dr. Donaldson states that she has no financial interest in the products or companies mentioned. She may be reached at KDonaldson@med.miami.edu.
1. Abrams D. Ophthalmic optics and refraction. In: Duke-Elder SS, ed. System of Ophthalmology. St. Louis: Mosby: 1970; 671-674.
2. Nichamin LD. Astigmatism control. Ophthalmol Clin N Am. 2006;485-493.
