Myopia is the most common ocular disorder worldwide, it is the leading cause of visual impairment in children, and its incidence is increasing rapidly.1,2 In 2010, an estimated 1.9 billion people (27% of the world’s population) were myopic, and 70 million of them (2.8%) had high myopia. These numbers are projected to rise to 52% and 10%, respectively, by 2050 (Figure).1
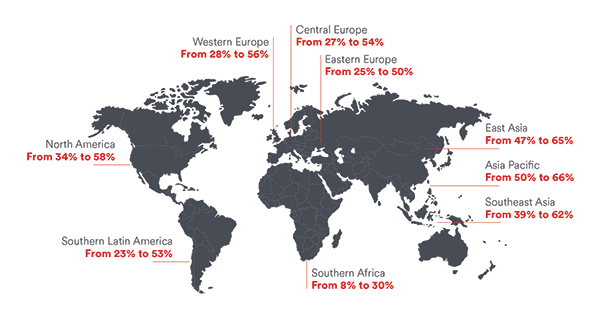
Figure. Current and projected 2050 myopia prevalence by region. (Reproduced with permission of Johnson & Johnson Vision and Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042.)
Myopia is a major public health concern in many East Asian countries, where the condition affects 80% to 90% of high school graduates. Of these individuals, 10% to 20% have sight-threatening pathologic myopia.2,3
Vision impairment related to myopia has a significant economic impact and a significant effect on quality of life regarding patients’ physical, emotional, and social functioning.4 Smith et al estimated the loss in world productivity caused by uncorrected myopic refractive error in 2004 to be 268.8 billion international dollars and the cost of addressing this problem to be US$28 billion.5
Pathologic myopia (prevalence 0.9%–3.1%) is particularly devastating.6,7 It confers an increased risk of cataract development, retinal detachment, glaucoma, and even blindness. The prevalence of choroidal neovascularization in affected individuals is reported to be 5.2% to 11.3%, and macular holes can occur in 6% to 8% of patients.6 The peripapillary regions are distorted by mechanical stretching of the globe in patients with increased axial lengths, and this can lead to glaucoma and visual field loss.8,9
The rapidly increasing incidence of myopia combined with its significant social and economic burdens have spurred research on causal factors, possible treatments, and efforts at prevention.
ETIOLOGY
The exact mechanism of an eye’s axial elongation is unknown. Genes alone do not account for the incidence of myopia, but they may determine how susceptible a given person is to environmental factors. Many twin, sibling, and parental myopia studies that have been conducted show a higher prevalence of myopia in identical versus fraternal twins and an increased risk of myopia with a higher number of myopic parents. Genetics alone also cannot account for the rapid rise of myopia in East Asia.10 Animal studies have shown that myopia can be induced in an eye that is deprived of proper vision; the longer the period of visual deprivation, the greater the induced myopia.10
The Consortium for Refractive Error and Myopia (CREAM), the largest international genome-wide study of myopia and refractive error ever conducted, found 24 genomic variations that were associated with a 10-fold increase in the prevalence of myopia.10 This suggests that myopia is multifactorial rather than the result of a single mechanism.
ENVIRONMENT
Increased outdoor activity has been shown to prevent the onset of myopia, with a possible dose-response relationship between time spent outdoors and the risk of incident myopia.11,12 Once someone begins to develop myopia, however, the time that he or she spends outdoors does not seem to halt its progression.11 Interestingly, simply being outside without engagement in a sport or activity has been shown to reduce the prevalence of myopia.13 This finding suggests that exposure to light may play a major role in myopia prevention. Light-induced release of dopamine, nitric oxide, and other neurotransmitters has been shown to inhibit axial elongation.10,12
There is mounting public concern that modern society’s dependence on computers and handheld technologies may be contributing to the increasing global incidence of myopia. Although axial length is temporarily increased with accommodation after just 10 to 30 minutes of near work,14,15 whether near work actually contributes to the rising incidence and prevalence of myopia remains controversial.16
CONTACT LENSES
It is theorized that visual input to the peripheral retina regulates adjacent scleral growth, and the relative peripheral hyperopic defocus in myopic eyes that remains despite wearing corrective myopic spectacles may be associated with elongation of the globe.17,18 Some have proposed using contact lenses that emphasize peripheral myopic defocus—and thus minimize hyperopic defocus—to help decrease myopic progression. In orthokeratology regimens, rigid contact lenses are used to flatten the central cornea, temporarily reducing myopic refractive error and inducing corneal swelling in the periphery, resulting in peripheral myopic defocus. A meta-analysis report by the AAO issued in April 2019 determined that orthokeratology may slow myopic progression in children and adolescents, with a potentially greater effect when treatment is initiated in patients who are 6 to 8 years of age.19 Serious safety concerns are associated with orthokeratology, however, such as the potential for blinding microbial keratitis associated with overnight contact lens wear.19,20
A study conducted in Hong Kong demonstrated 25% slower progression of myopia with specialty bifocal defocus-incorporated soft contact lenses than with single-vision soft contact lenses. Treatment effect correlated positively with the amount of time that the defocus-incorporated soft contact lenses were worn (see Technologies in Development).21
SPECTACLES
Another area of interest is children’s use of bifocal glasses to slow myopia by reducing accommodative lag and hyperopic defocus (see Technologies in Development).7 A 2002 study found that undercorrecting myopia enhanced rather than inhibited myopia progression, likely because image blur induces axial elongation.22 Some studies, including the 5-year results from the Correction of Myopia Evaluation Trial (COMET), have been unable to show a statistically significant difference in the effects of progressive addition versus single-vision lenses on myopia progression.23-25 Another study, however, demonstrated a 57% reduction in the rate of myopia progression after 3 years of using executive bifocals with a 1.50 D addition and 3.00 D base-in prism.26
ATROPINE
One area of great research interest is the use of atropine for myopia prevention (see Technologies in Development). This cycloplegic medication dilates the pupil and limits accommodation. The exact mechanism by which atropine works for myopia control is unclear, but the drug’s effect on muscarinic receptors in scleral fibroblasts is thought to slow axial elongation.27-30
In the Atropine for Treatment of Childhood Myopia (ATOM1) study, eyes treated with atropine 1% experienced reductions in refractive error change (-0.28 ±0.92 D) and axial elongation (-0.02 ±0.35 mm) over a 2-year period.25 The follow-up ATOM2 study showed similarly effective dose-dependent results with lower concentrations of atropine (0.5%, 0.1%, and 0.01%) and reductions in the side effects of glare and discomfort.28 Importantly, phase 2 of ATOM2 showed a rapid rebound increase in myopia when atropine was stopped for 12 months after the 24 months of study treatment; the group receiving the lowest concentration of atropine, 0.01%, had the least rebound effect. In phase 3 of ATOM2, children who experienced a rebound effect and whose myopia continued to progress were restarted on atropine 0.01%.
Ultimately it seems that, because patients in the 0.01% treatment arm had the lowest rebound effect, it may be the most effective dose for limiting overall myopia progression and axial elongation, according to Chia and colleagues.29 Based on their data, the authors suggested that a promising approach might be to use a stronger 0.5% concentration of atropine in the first year of treatment, followed by 0.01% the second year, to potentially limit rebound myopia progression.
The Low-Concentration Atropine for Myopia Progression (LAMP) study evaluated the safety and efficacy of eye drops containing atropine at 0.05%, 0.025%, and 0.01% compared to placebo over a 1-year period. All concentrations of the drug reduced myopia progression, but there was a concentration-dependent response. The 0.05% concentration was the most effective at controlling spherical equivalent progression and axial elongation.30
CONCLUSION
The incidence and prevalence of myopia are certainly on the rise, and increasing time spent indoors and on near work during childhood both may play a part. Fortunately, intense research efforts on prevention are ongoing, and several treatments hold promise for slowing or halting the progression of this potentially devastating condition.
Technologies in Development
Several companies are racing to develop innovative treatments to combat the myopia epidemic.
COOPERVISION
CooperVision released 4-year data from its MiSight 1-Day Soft Contact Lens Study in September 2018.1 The company’s dual-focus soft 1-day contact lenses are specially designed to slow the progression of juvenile-onset myopia (Figure 1). The lenses were shown to significantly and progressively reduce myopia progression and axial elongation over 4 years and even to have an effect on children who began treatment at an older age.2 MiSight lenses are already available in Canada and several European and Asian countries, but they have not yet been approved by the FDA.

Figure 1. MiSight 1-day soft contact lens.
Courtesy of CooperVision
EYENOVIA
In June, EyeNovia began enrolling patients in the US-based phase 3 multicenter, randomized, double-masked CHAPARONE study of MicroPine for progressive myopia. The study will enroll more than 400 children with the lowest exposure to topical atropine in a myopia-specific clinical trial to date, according to the company. Investigators will study the safety and efficacy of EyeNovia’s proprietary atropine topical microformulation (MicroPine) delivered by its Optejet dispenser (Figure 2). The company’s system uses piezo-print technology to allow children to self-administer 8 µL of atropine in order to minimize dose-related side effects.3
HOYA
Hoya is collaborating with a research team on the Defocus Incorporated Multiple Segments (DIMS) spectacle lens. The DIMS lens features a central optical zone that corrects refractive error and an array of microlenses around the central zone and extending to the midperiphery that produce constant myopic defocus. The team conducted a 2-year randomized clinical trial involving 183 Chinese children aged 8 to 13 years who had between -1.00 and -5.00 D of myopia and 1.50 D or less of astigmatism. Compared with children wearing single-vision (SV) lenses, the children wearing DIMS lenses experienced significantly less (52%) myopia progression. The DIMS lenses also slowed axial elongation by 62% compared to SV lenses. Seventeen of the 79 children (21.5%) in the DIMS group and six of the 81 children (7%) in the SV group experienced no myopia progression during the 2-year study.4
JOHNSON & JOHNSON VISION
In November 2018, the Singapore National Eye Centre, the Singapore Eye Research Institute, and Johnson & Johnson Vision announced a $26.35 million research collaboration to tackle the myopia epidemic. The stated goal of this public-private strategic partnership is to develop a deeper understanding of how the condition develops and progresses and how it may be intercepted. The effort is reportedly focused on creating predictive tools to identify people who may be at risk of developing high myopia, researching the underlying mechanisms of myopia, advancing novel therapies, and discovering and validating methods by which to prevent the onset of myopia and halt its progression.5
SIGHTGLASS VISION
SightGlass Vision is developing spectacle lenses to slow myopia progression in children. In January, the company began enrollment in the CYPRESS trial, a multicenter, double-masked, randomized controlled clinical trial at 14 clinical sites in the United States and Canada. The primary outcome measure will be myopia progression (changes in axial length and spherical equivalent refraction) over the course of 36 months.6
SYDNEXIS
Sydnexis is developing SYD-101, a proprietary drug for slowing myopia progression in children. In April, the FDA accepted the company’s investigational new drug application. In May, Sydnexis announced that it had dosed the first patients in the STAAR Study, a phase 3 multicenter, randomized, double-masked, placebo-controlled trial testing the safety and efficacy of two dosage strengths of SYD-101 in more than 800 children between the ages of 3 and 14 years.7

Figure 2. The Optejet dispenser.
Courtesy of EyeNovia
1. CooperVision releases four-year data on landmark MiSight 1 Day Contact Lens Study; pioneering approach slows myopia progression in children [press release]. Singapore: CooperVision; September 18, 2018. https://coopervision.com/our-company/news-center/press-release/coopervision-releases-four-year-data-landmark-misight-1-day. Accessed July 1, 2019.
2. Ruiz-Pomeda A, Pérez-Sánchez B, Valls I, et al. MiSight Assessment Study Spain (MASS). A 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):1011-1021.
3. Eyenovia enrolls first patient in phase III CHAPERONE Study for progressive myopia [news release]. New York: Globe Newswire; June 4, 2019. www.globenewswire.com/news-release/2019/06/04/1863931/0/en/Eyenovia-Enrolls-First-Patient-in-Phase-III-CHAPERONE-Study-for-Progressive-Myopia.html. Accessed Ju1y 1, 2019.
4. Lam CSY, Tang WC, Tse DY, et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial [published online ahead of print May 29, 2019]. Br J Ophthalmol. doi: 10.1136/bjophthalmol-2018-313739.
5. Singapore National Eye Centre, Singapore Eye Research Institute, and Johnson & Johnson Vision set sights on halting global myopia epidemic [news release]. Singapore: PRNewswire; November 12, 2018. www.prnewswire.com/news-releases/singapore-national-eye-centre-singapore-eye-research-institute-and-johnson–johnson-vision-set-sights-on-halting-global-myopia-epidemic-300747895.html. Accessed July 1, 2019.
6. SightGlass Vision multicenter trial to study novel eyeglasses to control nearsightedness for FDA approval [news release]. Palo Alto, CA: PRNewswire; January 10, 2019. https://prnmedia.prnewswire.com/news-releases/sightglass-vision-multicenter-trial-to-study-novel-eyeglasses-to-control-nearsightedness-for-fda-approval-300776509.html. Accessed July 1, 2019.
7. Sydnexis announces enrollment of first patients in phase 3 myopia STAAR Study of SYD-101 in children [news release]. Eyewire: May 2, 2019. https://eyewire.news/articles/sydnexis-announces-enrollment-of-first-patients-in-phase-3-myopia-staar-study-of-syd-101-in-children. Accessed July 1, 2019.
1. Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102(7):855-862.
2. Ma Y, Qu X, Zhu X, et al. Age-specific prevalence of visual impairment and refractive error in children aged 3-10 years in Shanghai, China. Invest Ophthalmol Vis Sci. 2016;57(14):6188-6196.
3. Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore. 2004;33(1):27-33.
4. Wong HB, Machin D, Tan SB, Wong TY, Saw SM. Visual impairment and its impact on health-related quality of life in adolescents. Am J Ophthalmol. 2009;147(3):505-511.e1.
5. Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ. 2009;87(6):431-437.
6. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(1):9-25.e12.
7. The impact of myopia and high myopia. Report of the Joint World Health Organization–Brien Holden Vision Institute Global Scientific Meeting on Myopia. University of New South Wales, Sydney, Australia, March 16-18, 2015. Geneva: World Health Organization; 2017. License: CC BY-NC-SA 3.0 IGO. https://www.who.int/blindness/causes/MyopiaReportforWeb.pdf. Accessed June 25, 2019.
8. Chihara E, Liu X, Dong J, et al. Severe myopia as a risk factor for progressive visual field loss in primary open-angle glaucoma. Ophthalmologica. 1997;211(2):66-71.
9. Ohno-Matsui K, Shimada N, Yasuzumi K, et al. Long-term development of significant visual field defects in highly myopic eyes. Am J Ophthalmol. 2011;152(2):256-265.e1.
10. Carr BJ, Stell WK. The science behind myopia. In: Kolb H, Nelson R, Fernandez E, Jones B, eds. Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center. https://webvision.med.utah.edu/book/part-xvii-refractive-errors/the-science-behind-myopia-by-brittany-j-carr-and-william-k-stell. Accessed June 25, 2019.
11. Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95(6):551-566.
12. French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58-68.
13. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279-1285.
14. Ghosh A, Collins MJ, Read SA, Davis BA, Chatterjee P. Axial elongation associated with biomechanical factors during near work. Optom Vis Sci. 2014;91(3):322-329.
15. Woodman EC, Read SA, Collins MJ, et al. Axial elongation following prolonged near work in myopes and emmetropes. Br J Ophthalmol. 2011;95(5):652-656.
16. Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children-a systematic review and meta-analysis. PLoS One. 2015;10(10):e0140419.
17. Smith EL III, Hung LF, Arumugam B. Visual regulation of refractive development: insights from animal studies. Eye (Lond). 2014;28(2):180-188.
18. Yeo A, Paillé D, Drobe B, Koh P. Myopia and effective management solutions. Points de Vue. https://www.pointsdevue.com/article/myopia-and-effective-management-solutions. Accessed July 8, 2019.
19. VanderVeen DK, Kraker RT, Pineles SL, et al. Use of orthokeratology for the prevention of myopic progression in children: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(4):623-636.
20. Kam KW, Yung W, Li GKH, Chen LJ, Young AL. Infectious keratitis and orthokeratology lens use: a systematic review. Infection. 2017;45(6):727-735.
21. Lam CS, Tang WC, Tse DY, Tang YY, To CH. Defocus incorporated soft contact (disc) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol. 2014;98(1):40-45.
22. Chung K, Mohidin N, O’Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42(22):2555-2559.
23. Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012;53(2):640-649.
24. Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, Hussein M; COMET Group. The Correction of Myopia Evaluation Trial (COMET): design and general baseline characteristics. Control Clin Trials. 2001;22(5):573-592.
25. Gwiazda JE, Hyman L, Everett T, et al. Five-year results from the Correction of Myopia Evaluation Trial (COMET). Invest Ophthalmol Vis Sci. 2006;47(13):1166.
26. Cheng D, Woo GC, Drobe B, Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol. 2014;132(3):258-264.
27. Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-2291.
28. Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354.
29. Chia A, Lu QS, Tan D. Five-year clinical trial on Atropine for the Treatment of Myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391-399.
30. Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113-124.




