The optimal tool for the correction of high myopia has long remained elusive, but recent efforts toward its discovery have been promising. The hope is that this trend continues until a solution that fully satisfies both the patient and the surgeon becomes available.
Attempts to correct high myopia began in the past century. In the late 1880s, Vincenz Fukala, MD, began extracting the clear crystalline lenses of young patients with high myopia. This research led to the development of two cornea-based methods for the correction of high myopia: (1) keratomileusis by José Barraquer, MD, and Jörg Krumeich, MD, and (2) epikeratophakia by Herbert E. Kaufman, MD, and Dr. Krumeich.
During epikeratophakia, the corneal epithelium was removed, and a lathed donor lenticule was placed onto the recipient cornea and secured with sutures until it healed into place. Thus, this treatment modality has been referred to as a living contact lens. The procedure is no longer routinely performed, given the advantages of modern laser refractive surgery.
One of the most researched tools for the correction of high myopia are phakic IOLs. Many surgeons and manufacturers are committed to developing the optimal lens solution. At the time of this writing, two types of lenses have been developed that are supported by long-term follow-up. This article examines their history and evolution. For the sake of simplicity, the lenses are described in terms of their positioning within the eye (Table).
ANTERIOR CHAMBER PHAKIC IOLS
The first phakic IOL for the correction of myopia was invented in 1953 by Benedetto Strampelli, MD. The Strampelli lens was a negative-powered IOL with a concave-convex shape designed for implantation in the anterior chamber. Ultimately, this IOL was associated with numerous complications, including endothelial failure, ovalization of the pupil, and fibrosis at the angle. All of these complications were attributed to the lens material, which was thick and inadequately finished. Further, incorrect sizing resulted in contact between the IOL and the structures of the angle, which had subsequent downstream consequences (Figure 1).
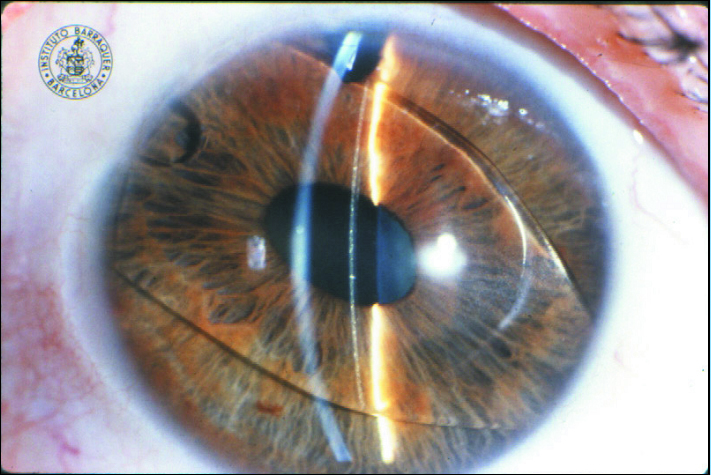
Figure 1. A rare photograph of Strampelli’s first phakic IOL.
(Courtesy of the Barraquer Institute in Barcelona)
Toward the end of the 1950s, similar lenses were developed by Dr. Barraquer and by Peter Choyce, FRCS, DOMS, MS. These IOLs were not particularly successful and were associated with the same types of complications as the Strampelli lens.1 Dr. Barraquer’s lens had a plate-haptic design that was similar to that of the Strampelli IOL. The lens’ radius and the rough material of the haptics frequently caused corneal edema in patients implanted with these IOLs.
In 1958, Dr. Choyce resumed Dr. Strampelli’s efforts to implant an IOL in the anterior chamber after implementing some changes to improve the tolerability of the IOL and to reduce side effects. Dr. Choyce used a refined material called Perspex CQ—also used by Sir Harold Ridley, MD, FRCSC—to reduce side effects related to impurities in the lens material. Additionally, Dr. Choyce changed the inner curvature ray of the IOL from 12 mm to 14 mm, flattening the overall curvature of the IOL and increasing the IOL’s distance from the endothelium. The goal was to reduce the incidence of corneal decompensation and dystrophies often related to the implantation of Dr. Strampelli’s and Dr. Barraquer’s lenses.
Dr. Choyce used thinner haptics, which were gentler on the angular structures. He reduced the thickness of the haptics from 0.91 mm to 0.50 mm in 1958 and from 0.50 mm to 0.25 mm in 1961. In 1959, he added an inferior ledge of 0.50 mm to reduce the incidence of iris prolapse. In his book Intra-Ocular Lenses and Implants, published in 1964, Dr. Choyce described 12 patients whose high myopia was corrected with a total of six monocular implants and six binocular implants. The complications observed included one case of iris prolapse that necessitated surgical intervention and five cases of pupil deformation (ovalization of the pupil) along the greatest axis of the lens. There was no evidence of secondary cataract.
In the 1980s, Georges Baikoff, MD; Jan Worst, MD; and Svyatoslav N. Fyodorov, MD, began experimenting with phakic IOLs for the correction of myopia. They explored the use of three different positioning sites—the anterior chamber, the iris, and the posterior chamber—and produced lenses in PMMA, silicone, collamer, and hydrophilic acrylic materials.
The anterior chamber phakic IOLs designed in 1986 by Merab Dvali, MD, PhD, and Dr. Baikoff were significantly more advanced than the lenses produced in the 1950s. The materials and shape of the haptics were more modern, and the lens was thinner, more flexible, better finished, and smoother. During this period, numerous models of lenses were produced, starting with the Baikoff ZB Lens and progressing to the ZB5M, the ZB5MF, and finally the NuVita lens.
The ZB Lens was produced of PMMA and had a Z-shaped haptic with four support points, a 25° haptic angle, and a 4.5-mm optic. In 1990, the lens was modified, and the newer model (ZB5M) had a smaller vaulting angle, a slimmer optic, and more flexible haptics. To increase biocompatibility, the ZB5MF lens was subjected to a process of fluorination. The final variation of the Baikoff lens was the NuVita MA 20 (Bausch + Lomb), a one-piece PMMA lens with a 5.0-mm optic plate. Despite the lens’ excellent design and manufacturing, it was associated with pupil deformation and defects at the angle and was eventually withdrawn from the market (Figure 2).
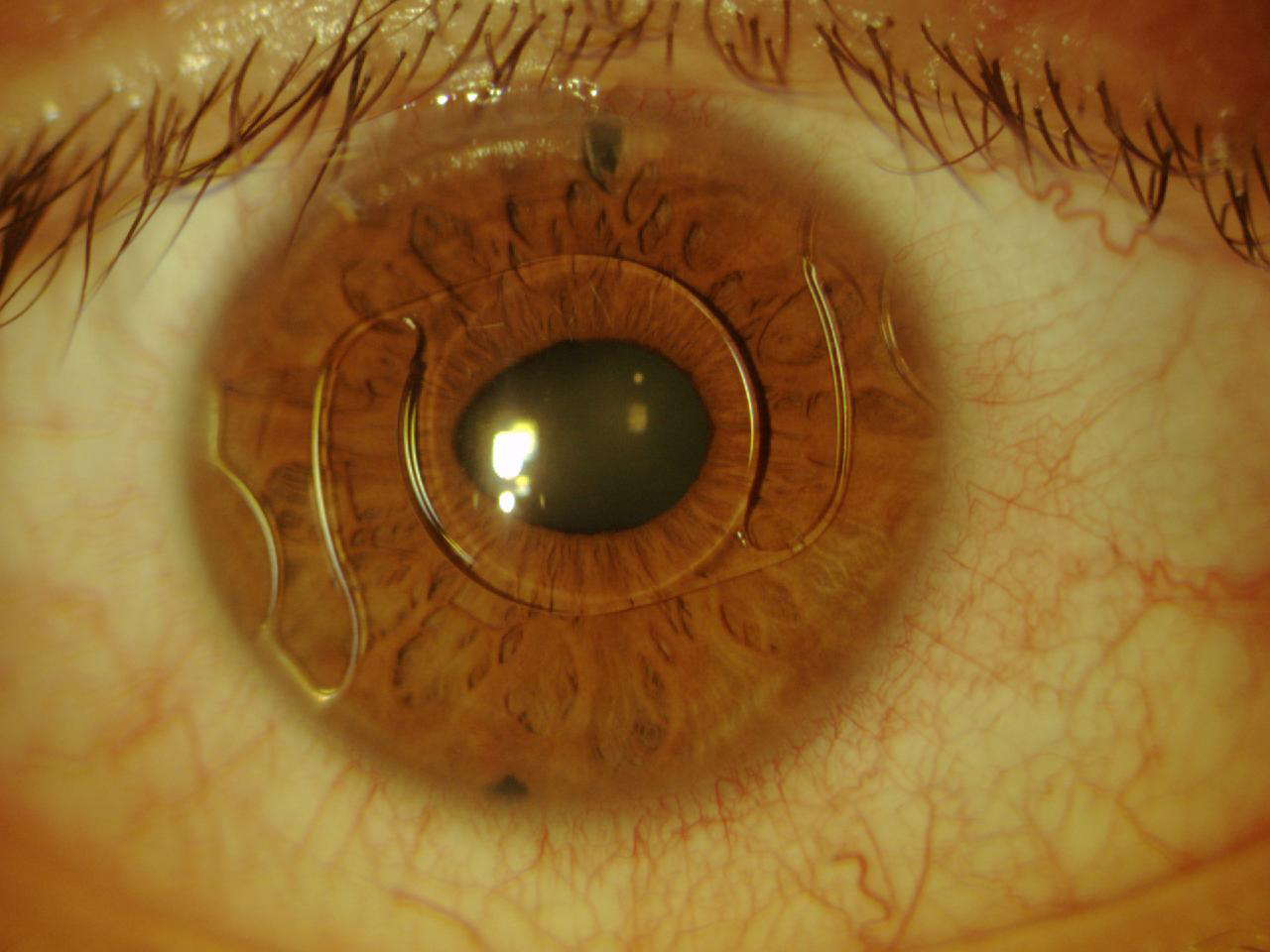
Figure 2. The NuVita IOL. One year after implantation, this IOL began to modify the pupil.
A number of other lenses for the anterior chamber were available during this period. Two of these designs—the ZSAL4 and ZSAL4-Plus (Morcher) and the Phakic 6 IOL (Ophthalmic Innovations International)—seemed to produce the best results. The ZSAL4 was a one-piece PMMA lens with a maximum length of 12.5 mm to 13.0 mm and a diameter of 5.5 mm. This lens was designed to reduce glare and night vision problems. The Z-shaped haptics had a 19° angle and were longer than standard haptics to minimize compression on the angular structures. This lens avoided the numerous complications associated with the Baikoff IOL, but ovalization and decentering of the pupil were still problematic.2 The Phakic 6 was also an angle-supported lens with an optic between 4.5 mm and 5.5 mm in diameter and a maximum length of 11.0 mm to 13.0 mm.3 This lens had a rigid optic and haptic design.
Another angle-supported lens was the Kelman Duet Implant Phakic IOL (Tekia), which launched in the early 2000s. This IOL was available in lengths of 12.0 mm, 12.5 mm, and 13.0 mm and was made of PMMA. It had a rigid optic and haptic, but the design was unique in that the optic and the haptics were separate. The haptics were designed with three support points that allowed the optic to be modified if the required refraction changed or if the dimensions of the haptics were inadequate. The lens was implanted by first inserting the haptics and successively injecting the foldable optic. The lens system was then assembled inside the eye (Figure 3).4 Other types of angle-supported IOLs included the GBR IOL (IOLtech Laboratories) and the Vivarte IOL (CIBA Vision). These lenses both had a flexible optic and rigid haptics. The Vivarte IOL was withdrawn from the market because it was shown to cause endothelial damage.5,6
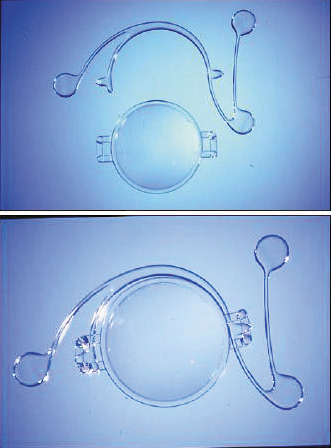
Figure 3. The Kelman Duet, Dr. Charles Kelman’s ingenious idea to design a two-piece lens that could be assembled inside the eye.
The ICare IOL (Cornéal Laboratories) was a one-piece hydrophobic acrylic IOL with flexible haptics and optic. It had four support points to prevent rotation and to reduce stress on the angle structures. The optic had a diameter of 5.75 mm, and the maximum overall diameter of the lens was 12.0 mm to 13.5 mm (Figure 4).
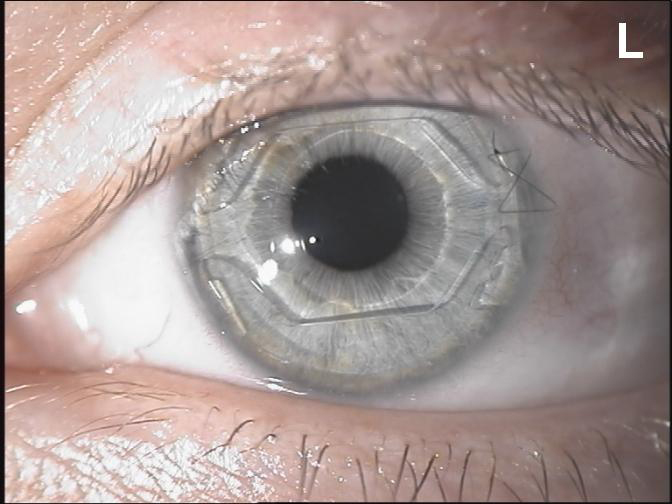
Figure 4. The ICare IOL had angle support and an optic disc surrounded by additional lens material that greatly increased the volume of the lens.
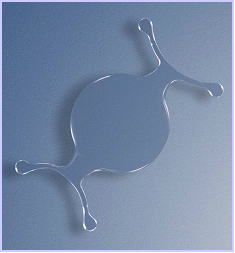
Figure 5. The Cachet was the last anterior chamber lens that was fairly successful, although only for a short period of time.
Another angle-supported lens was the AcrySof Phakic IOL (Cachet, Alcon; Figure 5). This one-piece lens had a flexible plate and haptics. The optic was shaped like a meniscus and had a diameter between 5.5 mm and 6.0 mm, with an overall length of 12.5 mm to 14.0 mm and a power range between -6.00 D and -16.50 D. The material of this lens was the same as that used for the AcrySof pseudophakic lens for implantation in the posterior chamber, a material that has shown high biocompatibility.7,8 However, it was determined that this lens also caused endothelial damage, and, thus, it did not receive FDA approval.
Overall, there have been problems with the anterior chamber lenses with angle support that were developed, and all of those that entered the market were subsequently withdrawn.
LENSES WITH IRIS ENCLAVATION
In the 1980s, a series of reports highlighted numerous complications associated with the implantation of anterior chamber phakic IOLs with angle support. As a result, researchers sought new types of lenses to reduce the adverse effects related to the presence of an IOL in the anterior chamber and to minimize trauma to the iridocorneal angle.
Consequently, an iris-fixated or iris-claw lens was produced from a Worst design developed in 1977. This lens had a biconcave optic plate produced in PMMA by compression molding and lathe cutting. Although initially designed for the correction of aphakia, this lens was later modified for the correction of high myopia in phakic patients. The advantage of enclavation was that one size fit all; the lens was designed so that the sizing would not require modification based on the different characteristics of the eye. Moreover, iris dilation was not compromised, and neither the structure of the angle nor the iris vasculature was altered.
In 1986, the Worst-Fechner iris-claw phakic IOL was developed with a biconcave PMMA optic. In 1991, the design was modified to a 5.0-mm lens with a convex-concave optic and a diameter of 8.5 mm. In 1998, the design was modified to incorporate a 0.87-mm vault anterior to the iris, and the option of a 6.0-mm optic was added. The name of the lens was changed to Artisan (Ophtec). In 2004, the lens received FDA approval under the name Verisyse Phakic IOL (Abbott Medical Optics, now Johnson & Johnson Vision) and a power of -5.00 D to -20.00 D. In Europe, a model was available with a spherocylindrical optic (toric model) and cylinder powers of up to 7.50 D.9-11
The Artiflex lens (Ophtec, not available in the United States) was subsequently designed and produced. This IOL is based on the Artisan platform but has a 6.0-mm optic plate made of flexible silicone and a convex-concave optic. The diameter of the lens is 8.5 mm, and it is available in powers ranging from -2.00 D to -14.50 D. The IOL can be inserted through a 3.2-mm self-sealing incision and can optimize the astigmatism induced by the incision (Figure 6).12
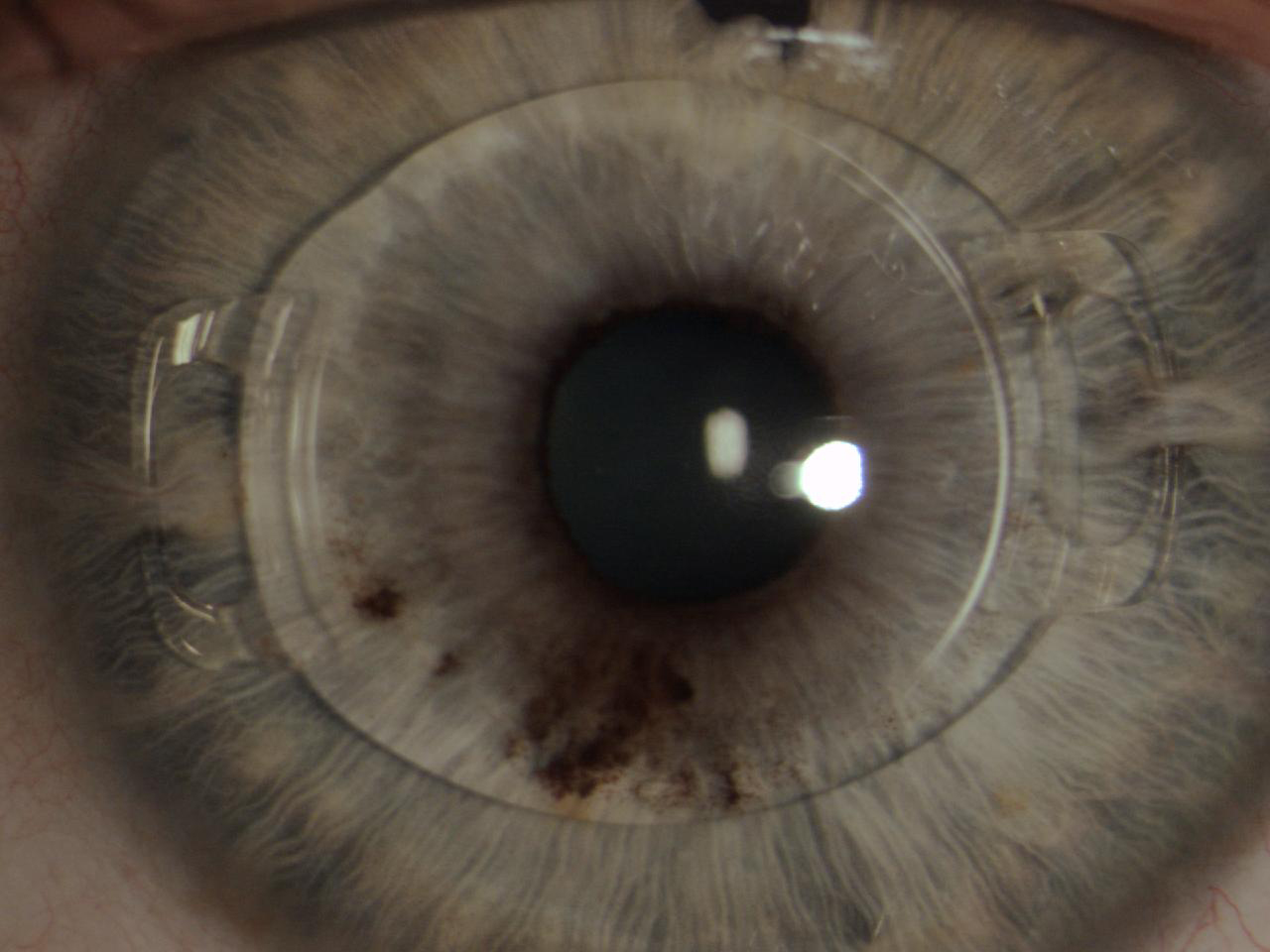
Figure 6. The Artiflex, a well-enclaved IOL. Iridectomy was performed.
POSTERIOR CHAMBER PHAKIC IOLS
The concept of implanting IOLs in the posterior chamber was a valid alternative for phakic eyes because these lenses are positioned at a safe distance from the endothelium and iridocorneal angle. They were also cosmetically appealing because they were visible only with careful examination.
The first posterior chamber IOL was developed in 1986 by Dr. Fyodorov. Initially, the lens had a collar button arrangement, with the optic in the anterior chamber and the haptics positioned in the posterior chamber behind the iris.13 The Chiron Adatomed phakic IOL was also designed for implantation in the posterior chamber. This silicone-plate haptic IOL had a length of 10.5 mm to 12.5 mm and a circular optical zone that was 5.5 mm in diameter. The Chiron lens was associated with an increased incidence of anterior chamber inflammation and cataract formation, and production was stopped as a result.14
Next, the Phakic Refractive Lens (CIBA Vision) was developed for posterior chamber implantation. This one-piece hydrophobic silicone IOL was designed to float on the crystalline surface behind the iris, with the haptics resting on the zonules. It was available in two sizes and had a power range from -3.00 D to -20.00 D. However, production of the Phakic Refractive Lens was abandoned because the lens induced zonular dehiscence with subluxation in the vitreous chamber (Figure 7).15
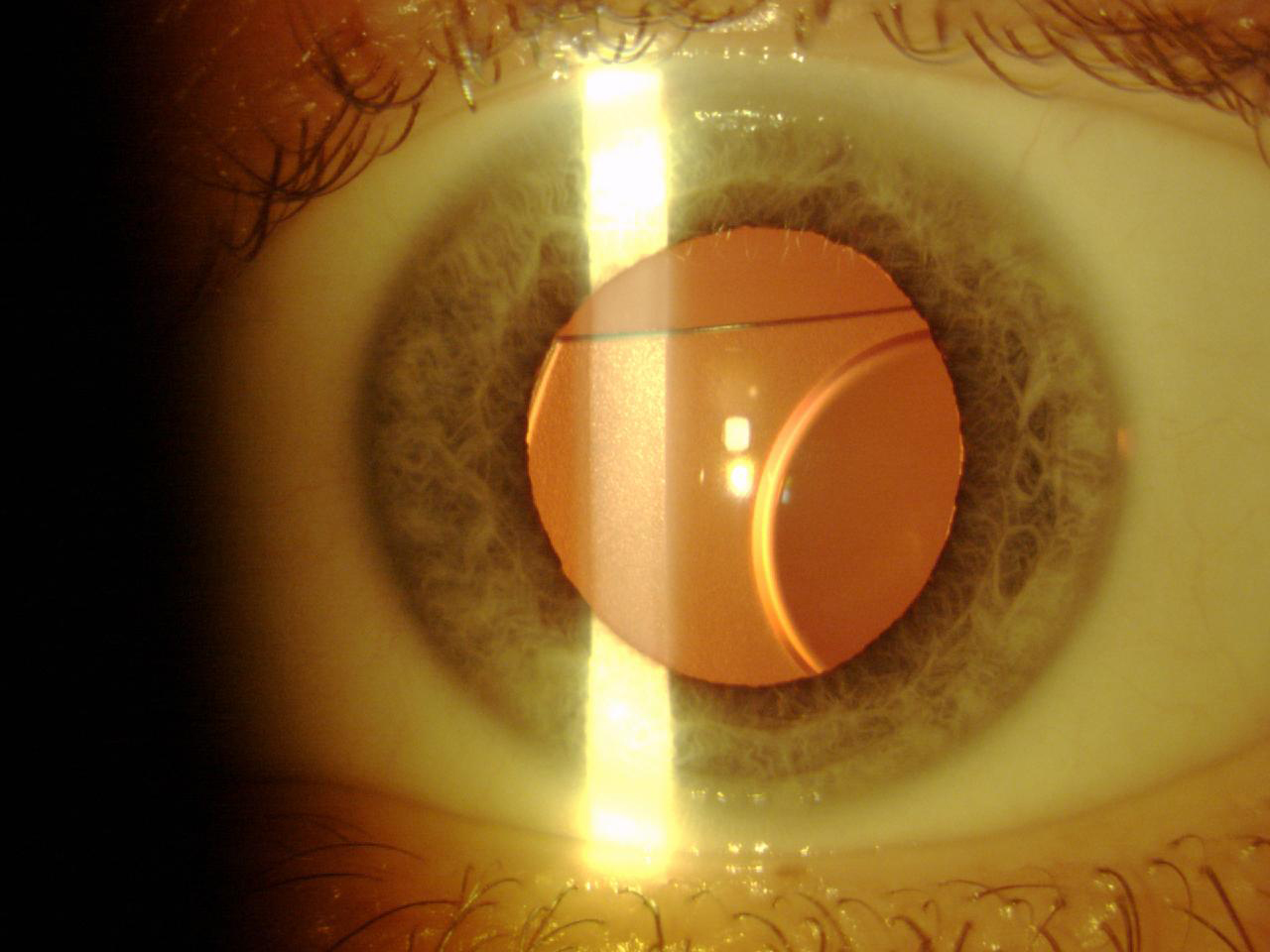
Figure 7. A Phakic Refractive Lens dislocated 2 years after surgery.
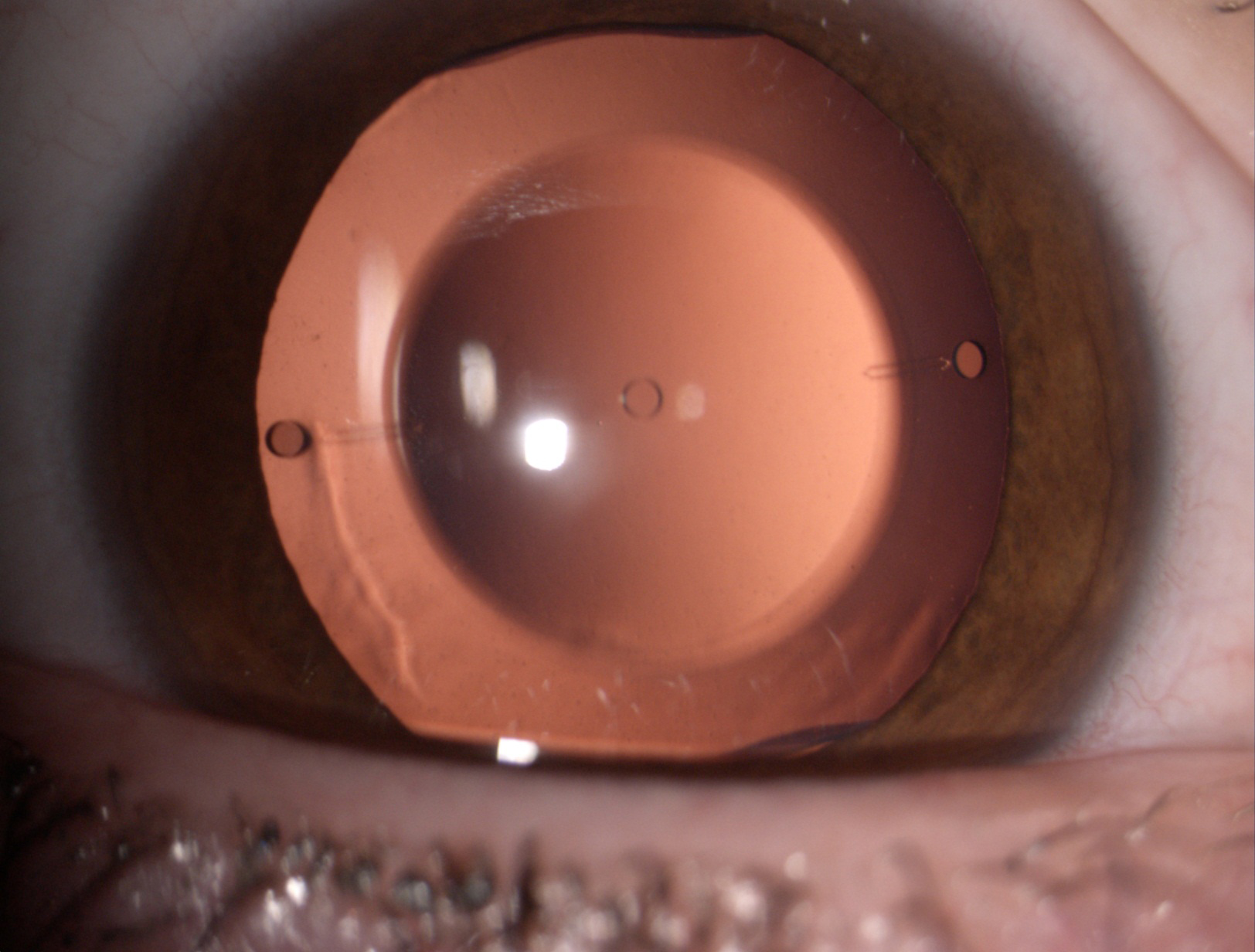
Figure 8. The Visian ICL phakic lens.
WATCH IT NOW
CL Removal at Cataract Surgery Bennett Walton, MD, MBA
Explanting an Anterior Chamber Phakic IOL Bennett Walton, MD, MBA
CONCLUSION
The Visian ICL and Artisan/Verisyse IOL are the only successes of a long history of attempts to correct high myopia and astigmatism. Their low incidence of complications correlated to their high refractive quality indicates that these types of lenses are valid options for the correction of high refractive errors when excimer laser surgery is not indicated, as in patients with thin corneas.
1. Fechner PU, Strobel J, Wichmann W. Correction of myopia by implantation of a concave Worst-iris claw lens into phakic eyes. Refract Corneal Surg. 1991;7:286-298.
2. Pérez-Santonja JJ, Alió JL, Jiménez-Alfaro I, Zato MA. Surgical correction of severe myopia with an angle-supported phakic intraocular lens. J Cataract Refract Surg. 2000;26:1288-1302.
3. Yu A, Wang Q, Xue A, et al. Comparison of contrast sensitivity after angle-supported, iris-fixated and posterior chamber phakic intraocular lens implantation for high myopia. Ophthalmologica. 2008;222:53-57.
4. Alió JL, Piñero D, Bernabeu G, et al. The Kelman Duet phakic intraocular lens: 1-year results. J Refract Surg. 2007;23:868-879.
5. Javaloy J, Alió JL, Iradier MT, et al. Outcomes of ZB5M angle-supported anterior chamber phakic intraocular lenses at 12 years. J Refract Surg. 2007;23:147-158.
6. Van Cleynenbreugel H. Late postoperative complications of backward implantation of a Vivarte phakic intraocular lens. J Cataract Refract Surg. 2007;33:1474-1476.
7. Oshika T, Nagata T, Ishii Y. Adhesion of lens capsule to intraocular lenses of polymethylmethacrylate, silicone, and acrylic foldable materials: an experimental study. Br J Ophthalmol. 1998;82:549-553.
8. Linnola RJ, Sund M, Ylonen R, Pihlajaniemi T. Adhesion of soluble fibronectin, laminin, and collagen type IV to intraocular lens materials. J Cataract Refract Surg. 1999;25:1486-1491.
9. Bartels MC, Saxena R, van den Berg TJ, et al. The influence of incision-induced astigmatism and axial lens position on the correction of myopic astigmatism with the Artisan toric phakic intraocular lens. Ophthalmology. 2006;113:1110-1117.
10. Dick HB, Alió J, Bianchetti M, et al. Toric phakic intraocular lens: European multicenter study. Ophthalmology. 2003;110:150-162.
11. Dick HB, Tehrani M, Aliyeva S. Contrast sensitivity after implantation of toric iris-claw lenses in phakic eyes. J Cataract Refract Surg. 2004; 30:2284-2289.
12. Tehrani M, Dick HB. Short-term follow-up after implantation of a foldable iris-fixated intraocular lens in phakic eyes. Ophthalmology. 2005;112:2189-2195.
13. Jiménez-Alfaro I, Benítez del Castillo JM, García-Feijoó J, et al. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2001;108:90-99.
14. Marinho A, Neves MC, Pinto MC, Vaz F. Posterior chamber silicone phakic intraocular lens. J Refract Surg. 1997;13:219-222.
15. Donoso R, Castillo P. Correction of high myopia with the PRL phakic intraocular lens. J Cataract Refract Surg. 2006;32:1296-1300.




