Patient expectations of achieving the targeted correction with toric IOLs have risen greatly. Simultaneously, the tools available to calculate and deliver on these expectations have improved. One such improvement involves the incorporation of posterior corneal astigmatism (PCA) into our measurements. The posterior cornea has long been known to affect total corneal astigmatism, so the calculations for toric IOLs have evolved to include PCA.
Recently, I conducted a study to assess the impact of including PCA into toric IOL power calculations. This article outlines that study and its findings.
PURPOSE AND METHODS
The purpose of this study was to determine if the online Barrett Toric Calculator, which accounts for the total corneal astigmatism—both anterior and posterior—provided more accurate toric IOL power outcomes than traditional calculation methods that accounted for only anterior corneal astigmatism.
The single-arm, unmasked, nonrandomized prospective study was conducted in patients undergoing cataract surgery with toric IOL implantation. The primary endpoint was the mean residual refractive cylinder with the Barrett Toric Calculator compared with the expected mean residual refractive cylinder using the previous Alcon Toric IOL calculator (theoretical back-calculations). Secondary endpoints included the distribution of residual refractive cylinder at 2 months, distance BCVA, UCVA at target distance, manifest refraction, and the percentage of patients with 0.50 D of refractive cylinder or less at 2 months postoperatively. A subgroup analysis evaluated residual refractive cylinder by axial length (eyes < 22 mm, between 22 and 26 mm, and > 26 mm), average residual refractive cylinder for each group, and percentage of eyes within 0.25 D, 0.75 D, and 1.00 D.
Clinical outcomes were analyzed 2 months postoperatively. We evaluated the distribution of the residual refraction (sphere and cylinder) in eyes undergoing cataract surgery with toric IOL implantation based on a lens suggestion from the Barrett Toric Calculator. The residual refractive cylinder achieved was compared with back-calculated theoretical results previously determined by an Alcon Toric IOL calculator.
Keratometry (K) values were obtained using the Lenstar LS900 (Haag-Streit). Placido disc–based topography was performed with the Atlas (Carl Zeiss Meditec) and/or OPD-Scan (Nidek). We used axial maps to review the regularity of the corneal astigmatism and compared the K values against the Lenstar calculations to ensure accuracy of the magnitude of astigmatism (within 0.20 D) and meridian (within 3°) between devices.
To estimate the effects of using an old calculator, we subtracted the effect of the current IOL from the existing refraction and added back in the IOL suggested by older calculator. This produced a new estimated refractive cylinder, allowing the vector difference between the two refractive cylinders (current/new) to be determined (Figure 1).
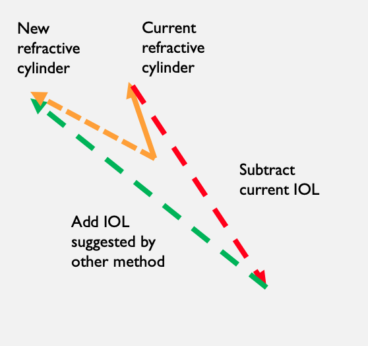
Figure 1. Vector math used to evaluate calculation methods.
CRITERIA
Inclusion criteria included patient age of 30 years or older; regular corneal astigmatism of 0.75 D to 5.00 D; and potential postoperative visual acuity of 20/25 or better. Exclusion criteria included amblyopia; previous radial keratotomy or other corneal surgery (eg, LASIK or PRK); previous anterior or posterior chamber surgery (eg, vitrectomy or laser peripheral iridotomy); diabetic retinopathy; macular pathology (eg, epiretinal membrane or age-related macular degeneration); pupil abnormalities (eg, corectopia); history of retinal detachment; an acute or chronic disease or illness that would increase operative risk or confound the results of the investigation; corneal pathology (eg, opacities, epithelial basement membrane dystrophy, and Fuchs dystrophy); and keratoconus or irregular astigmatism.
RESULTS
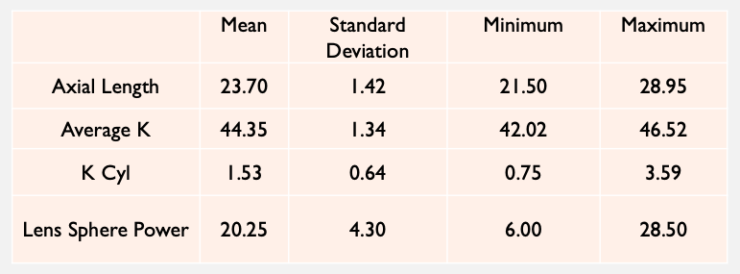
Basic demographics and preoperative data. A total of 40 eyes of 28 patients (17 women and 11 men) were included in the analysis (Table 1). Mean patient age was 68 ±9 years (range, 46–88 years).
TABLE 1. PREOPERATIVE PATIENT DATA (N = 40)
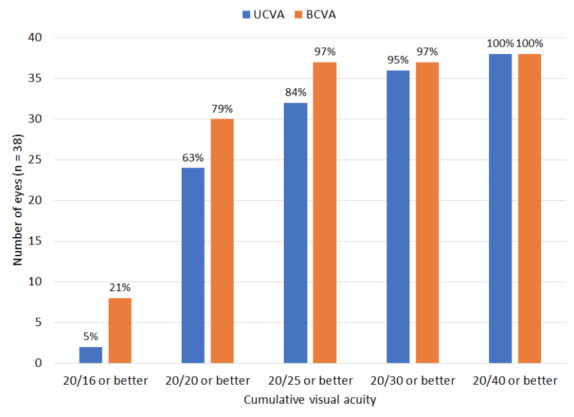
Of 38 eyes (two eyes with monovision were excluded), 84% (n = 32) had UCVA of 20/25 or better (Table 2). All but two eyes had UCVA within 1 line of their BCVA (95%; n = 36). Of all 40 eyes, 85% (n = 34) were within 0.25 D of the intended correction, and 100% (n = 40) were within 0.50 D of the intended correction.
TABLE 2. VISUAL ACUITY OUTCOMES
A proposed analysis by axial length was not meaningful, as there were too few eyes in the extreme groups. Three eyes had axial lengths less than 22 mm, 34 eyes had axial lengths between 22 mm and 26 mm, and three eyes had axial lengths greater than 26 mm. There was no evidence of correlation between axial length and residual refractive cylinder.
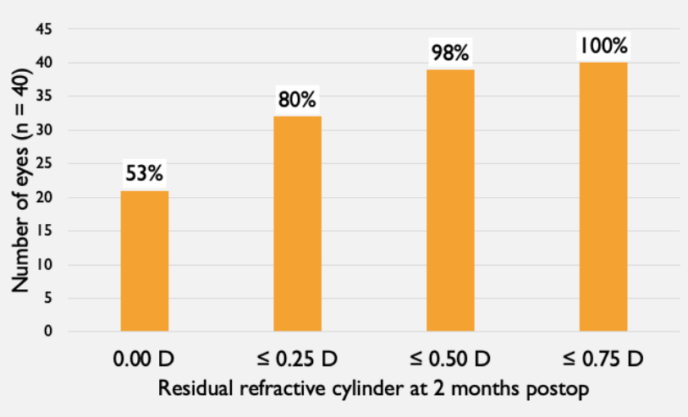
At 2 months postoperatively, lens orientation was not measured in two eyes (patient preference not to be dilated). Of the 38 that were measured, 95% were within 5° of the intended orientation, 5% were between 5° and 10° of the intended orientation, and no eyes were more than 10° from the intended orientation. Only one out of 40 eyes had more than 0.50 D of residual refractive cylinder (0.75 D; Table 3).
TABLE 3. RESIDUAL REFRACTIVE CYLINDER AT 2 MONTHS
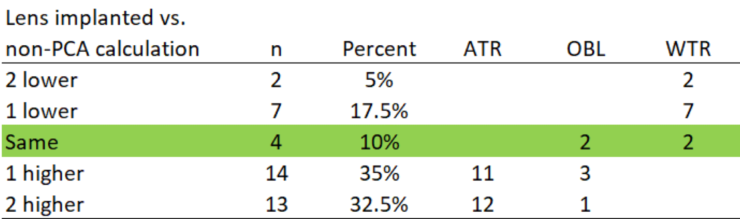
Comparing calculation methods. Algorithms accounting for PCA suggest lowering power with the rule and raising power against the rule. This was consistent with our observations (Table 4). In 37.5% of cases, the change was two cylinder powers (eg, T3 to T5). The potential reasons for this include: (1) PCA was not incorporated, (2) the legacy calculator would not have suggested flipping an axis, and (3) there was a study bias toward leaving patients with the rule.
TABLE 4. TORIC IOL IMPLANTED COMPARED TO NON-PCA CALCULATION
When evaluating residual refractive cylinder at 2 months, we found that back-calculated values tended to be about 0.20 D higher (Table 5). Non-PCA values appeared similar to what was reported in the literature before PCA was accounted for (~50% within 0.50 D). As shown in Table 6, the Barrett difference is residual cylinder versus expected cylinder from calculator. The non-PCA difference is back-calculated residual cylinder versus expected cylinder from the non-PCA calculator.
TABLE 5. RESIDUAL REFRACTIVE CYLINDER AT 2 MONTHS WITH BACK-CALCULATED RESULTS FOR NON-PCA CALCULATOR
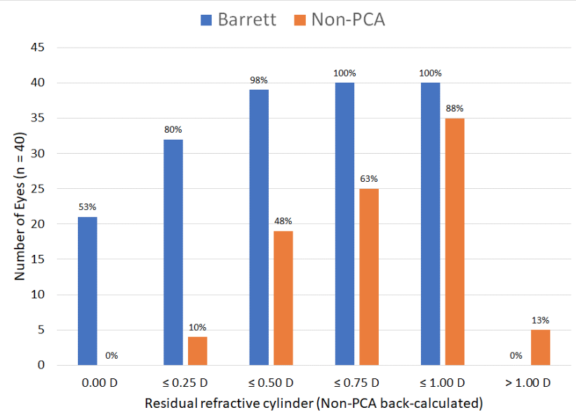
TABLE 6. DIFFERENCE FROM EXPECTED RESIDUAL REFRACTION
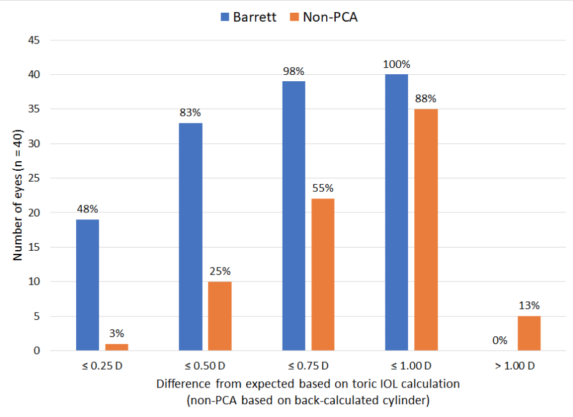
The differences in Tables 5 and 6 are pretty substantial, but the selection criteria (erring toward with the rule) and the fact that lens cylinder choices varied by more than two in about one-third of cases make the results quite believable. The non-PCA calculator was biased toward never flipping an axis, so expected residual refractions were always quite high.
DISCUSSION
Online toric IOL calculators that incorporate the Barrett algorithm—which accounts for PCA—provide more accurate refractive outcomes than toric calculators that account for only anterior corneal astigmatism. Accurate preoperative diagnostics to identify corneal astigmatism are essential. Limitations of this study include its small sample size and short-term (2 months) follow-up.



