CASE PRESENTATION
A 67-year-old Ethiopian woman presents with a complaint of a 1-month decrease in vision in her right eye. She suspects the problem is the result of a recent fall.
The patient underwent cataract surgery several years ago. Examination shows inferior corneal edema and a dislocated three-piece IOL, with optic capture inferiorly and the inferior haptic in the anterior chamber (Figure). Her visual acuity is 20/400, and there is no improvement with refraction. OCT imaging of the retina is normal.
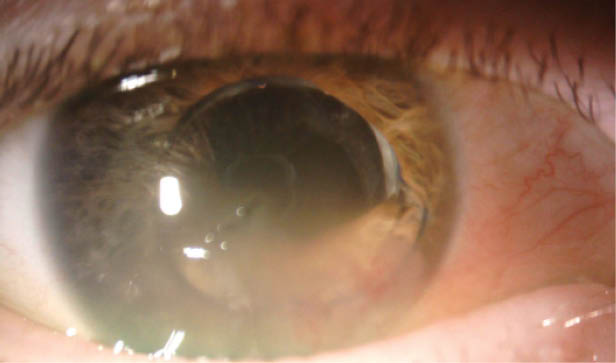
Figure | Corneal edema inferiorly and optic capture of a three-piece IOL, the inferior haptic of which is in the anterior chamber.
How would you approach this case? Would you attempt to reposition the IOL with or without iris suturing? Would you exchange the IOL? Would you consider combining intervention with Descemet membrane endothelial keratoplasty (DMEK) or Descemet-stripping endothelial keratoplasty?
—Case prepared by Audrey R. Talley Rostov, MD

JORGE L. ALIÓ, MD, PHD, FEBOPHTH
In all likelihood, complications occurred during this patient’s cataract procedure, and her current presentation has no connection with the trauma that she described. The original surgeon probably mistakenly placed the lens haptic in the anterior chamber, as the presence of iridocapsular synechiae suggests. The presence of the haptic in the anterior chamber has led to chronic corneal decompensation, and the condition probably precipitated recently.
The patient requires a corneal transplantation procedure, preferably DMEK. First, however, I would try to resolve the problem of the IOL because this surgery often causes inflammation. In the OR, with the patient under local retrobulbar anesthesia, I would de-epithelize the cornea and administer topical glycerol 20% to clear it for about 5 minutes. I would dissect the iridocapsular synechiae to study how extensive they are in order to replace the inferior IOL haptic in the sulcus. This maneuver can be traumatic and can lead to inadequate haptic support in the sulcus, in which case a 9-0 polypropylene suture, located inferiorly behind the iris, would be needed to provide support. I would constrict the pupil and execute tractional centripetal maneuvers at the inferior iris as needed with Alió MICS forceps (MicroSurgical Technology) to fully detach the iris from the synechiae and create a round pupil. At the conclusion of the case, I would instill 0.1 mL of triamcinolone in the anterior chamber and 0.9 mL in the orbital floor to prevent anterior segment inflammation.
Once the eye is quiet, the pupil is round, and the outcome was successful, I would proceed with DMEK. I have found that, in some cases, once the anatomy has been restored, corneal transparency returns and DMEK is no longer necessary. More often, however, DMEK is finally required.

BERNARDO DE PADUA SOARES BEZERRA, MD
This case presents a few challenges, including repositioning or replacing the IOL and addressing corneal endothelial decompensation and iris damage. This is most likely a case of posttraumatic subluxation of a three-piece IOL. The timing of surgery is important here. I would look for anterior segment inflammation and uveitis-glaucoma-hyphema syndrome. Media opacity from focal corneal edema must also be considered. Intervention should happen as soon as intraocular inflammation is under control, given the ever-growing risk of corneal decompensation and reduced visibility during surgery.
Less would be more in this case. In an initial procedure, I would only reposition or replace the IOL. I would prefer a superior approach to avoid constructing the wound in the area of corneal edema. I would fill the anterior and posterior chambers with OVD using a soft-shell technique to prevent further endothelial cell loss. I would viscodissect the IOL haptics from the iris and reposition the three-piece IOL in the sulcus by rotating and directing the haptics clockwise to the supraciliary space. The Figure shows sufficient capsular bag support for sulcus placement. For IOL replacement, power calculations should consider standard keratometry values with a slightly myopic target and sulcus implantation.
The two other problems can be managed secondarily if they become clinically significant, with endothelial keratoplasty after appropriate medical management of the corneal decompensation. I would prefer DMEK, although it could be technically challenging in a posttraumatic eye. If, after the first procedure the patient had significant glare or light sensitivity that was determined to be due to a focal iris defect or large corectopic pupil, I would perform a pupilloplasty or iris implant.

MICHAEL D. GREENWOOD, MD
I would assume that trauma from the fall caused dislocation of the haptic and a decrease in vision. Perhaps more important, the optic is captured in the bag, but the haptic is out of the bag, which may suggest complicated cataract surgery. These findings have me concerned about lack of stability of and support for the bag-zonular system.
I would plan for multiple scenarios in the OR. After maximal pupillary dilation, I would identify and remove any vitreous that was present. Next, I would check the stability of the IOL-zonular system. If I found it stable for 360º and no vitreous present, then I would reposition the haptic in the sulcus and ensure that the other haptic was well positioned, most likely in the sulcus. If the system is stable but a focal area of weakness is present inferiorly, then I would likely place a single suture inferiorly to fixate the complex to the iris. If the trauma had dislocated the haptic, caused significant zonular loss in that area with protruding vitreous, and destabilized the IOL complex, I would perform scleral fixation with the Yamane technique. It would be possible to use the existing IOL, but my preference would be to exchange it for a three-piece CT Lucia IOL (Carl Zeiss Meditec), which has haptics I find to be a bit more forgiving in the Yamane technique.
Because the edema is likely related to the displaced haptic and has been present for only a short period, I would not plan on performing Descemet-stripping endothelial keratoplasty simultaneously. The edema should clear after IOL surgery, but the patient may require corneal transplantation in the future.

FRANCIS W. PRICE JR, MD
One does not know when the IOL dislocated. It might have occurred with the fall or at the time of cataract surgery, with corneal edema just now affecting the patient’s vision. In the Figure, it is also unclear whether the posterior capsule is intact or whether it has a capsulotomy or a new or old rupture. The edges of the optic are sharp, so placing the lens in the sulcus could lead to chafing of the overlying iris. Ideally, if the lens recently dislocated out of the bag, the bag may be dissected open with OVD and the lens repositioned in the bag, but that possibility is doubtful. Placing the haptics behind the iris with capture of the optic behind the capsule would be possible if the lens can be freed from synechiae and the iris freed from the capsule (if present). In cases such as this one, however, the iris is often firmly bound to the capsule, and dissection is difficult or impossible.
A pars plana vitrectomy would allow opening of an unopened capsule for optic capture and removal of any associated vitreous prolapse. If the haptic is too scarred into the iris to be removed, then amputation from the IOL may be necessary, along with an IOL exchange for a three-piece CT Lucia IOL. If sufficient and stable capsular support cannot be achieved, then the lens can be fixated to the sclera with the Yamane technique to avoid old scleral injuries.
I would first address the IOL alone and see if the cornea cleared before recommending DMEK. Often these corneas clear after the inflammation and offending IOL are treated.

EVAN D. SCHOENBERG, MD
Preoperatively, I would prepare for the possibility of simple repositioning and fixation of the dislocated three-piece IOL or for IOL exchange as a last resort. I would obtain old records for the patient’s IOL power, if possible, or measure the eye and the keratometry of the fellow, nonedematous cornea, if needed, so as to order a backup lens suitable for transscleral fixation (CT Lucia 602) in case exchange is needed.
The existing IOL appears to have dislocated from the sulcus, and it is likely that the posterior capsule is open. I would therefore be prepared for vitrectomy. Given the extent of corneal edema throughout most of the inferior cornea and the central bullous changes, I doubt the cornea will clear without endothelial keratoplasty. I would discuss with the patient the options of simultaneous IOL repositioning and Descemet-stripping automated endothelial keratoplasty (DSAEK) and of sequential IOL repositioning followed 1 month later by either DSAEK or DMEK, depending on candidacy.
The first priority at the start of surgery is to achieve good visualization. I would inject 0.2 mL of triamcinolone into the anterior chamber. If I saw vitreous anterior to the IOL, I would fashion a small peritomy, use a microvitreoretinal blade (Accutome) to enter through the pars plana, and perform a limited pars plana vitrectomy with anterior chamber infusion. I would suture the sclerotomy with an absorbable suture. Then I would instill trypan blue ophthalmic solution (VisionBlue, Dutch Ophthalmic USA) to stain the capsule. Next, I would gently elevate the posterior half of the IOL with OVD and place iris hooks at the 5, 6:30, and 7:30 clock positions.
If the sulcus appeared to be stable, I would rotate the IOL back into place and then remove the hooks. If the sulcus was minimally unstable in an isolated area inferiorly, I would move the inferior haptic posterior to the iris, remove the hooks, suture the inferior haptic to the midperipheral iris with a 9-0 polypropylene suture on a CIF-4 needle using a Siepser knot, and prolapse the optic posteriorly. If I discovered that the sulcus was grossly unstable or the IOL was damaged, I would exchange the existing IOL for the CT Lucia 602 backup lens and perform double-needle transscleral fixation with the Yamane technique.
If the patient wanted her vision restored with a single surgical procedure, I would choose simultaneous DSAEK to manage the corneal edema. Although, in general, I prefer DMEK for its better visual results and lower rejection rate than even ultrathin DSAEK, graft unfolding in DMEK can require many adjustments to the anterior chamber depth, which could destabilize the newly repositioned IOL. Additionally, I would be concerned that, after trauma to the pupillary margin, the pupil might not constrict sufficiently to allow the iris to protect the graft endothelium from the IOL. I would instill carbachol intraocular solution (Miostat, Novartis) to constrict the pupil. Next, I would use intraocular scissors to fashion a small inferior peripheral iridotomy to prevent pupillary block from the air bubble placed at the conclusion of surgery because I would not want to redilate the pupil immediately. An anterior chamber maintainer would help ensure chamber stability during DSAEK graft insertion.
If visual improvement after at least 1 year ultimately halted at around 20/30, if no explanation for vision loss other than the DSAEK stroma-stroma interface was seen, and if the pupillary sphincter was functional or the patient was willing to undergo pupillary cerclage to permanently constrict the pupil, a regraft with DMEK could be considered at that time.
If the patient was willing to undergo two surgeries for the possibility of achieving 1 to 2 lines of better vision on average, I would explain that, once the IOL was repositioned and she had healed for a few weeks, we could determine whether she is a candidate for DMEK or DSAEK. I would then proceed accordingly.

RUSSELL SWAN, MD
Given that neither the optic nor the inferior haptic is in the capsular bag complex, this three-piece IOL was likely previously placed in the sulcus. An open posterior capsule and associated corneal decompensation increase the complexity of the case.
I would perform a retrobulbar block and start by marking the sclerotomy sites for the Yamane technique. I would place a 25-gauge trocar via an inferior temporal incision through the pars plana and then perform a limited anterior vitrectomy with anterior infusion to ensure the absence of vitreous. Through a temporal 2.75-mm incision and paracentesis, I would secure the IOL with MST microforceps and cut the IOL with MST IOL scissors (both instruments from MicroSurgical Technology) three-quarters of the way across the optic. I would externalize the IOL through the main incision. Next, I would use the Yamane technique for scleral fixation of a three-piece CT Lucia IOL of the appropriate power based on the previous IOL and residual refractive error prior to corneal decompensation.
Given the degree of corneal decompensation, I would discuss with the patient preoperatively the option of a staged procedure, but my recommendation would be to combine IOL exchange with ultrathin DSAEK because of the vitrectomy. At the conclusion of the IOL procedure, I would perform standard DSAEK using the Endoserter (CorneaGen) for controlled delivery of the graft into the anterior chamber and place an air bubble at the conclusion of the case. I believe this approach has the greatest chance of long-term success with the fewest interventions for this 67-year-old woman.

WHAT I DID: AUDREY R. TALLEY ROSTOV, MD
I proceeded with the case using a peribulbar block. I placed one paracentesis incision temporally and additional ones at the 12 and 6 clock positions. I performed an injection of Healon GV (Johnson & Johnson Vision) and explored the position of the IOL. I separated the synechiae with viscodissection and a cyclodialysis spatula as well as with microforceps (MicroSurgical Technology) and horizontal scissors.
No vitreous was present in the anterior chamber. I repositioned the three-piece IOL and rotated it 90º into the sulcus. I irrigated out the OVD. I was prepared to perform an IOL exchange or to suture the haptic to the iris with a polypropylene suture on a CIF-4 needle, but the IOL appeared to be stable after rotation. I instilled carbachol intraocular solution and hydrated the paracentesis incisions.
Since the surgical intervention, I have been observing the patient. Thus far, she has not required further IOL manipulation or DMEK surgery, but I am prepared to perform these procedures in the future if required.