From plugs to pumps, innovative drug delivery systems are in the pipeline to aid us in overcoming one of the biggest challenges in glaucoma care: lack of compliance.
PLUGS AND INSERTS
Ocular Therapeutix has been working on a drug-eluting punctum plug that contains travoprost. The travoprost molecules are released by hydrolysis from microspheres that are suspended in a biodegradable hydrogel rod. This plug can provide sustained release of travoprost over 2 to 3 months. The company also has a similar product for delivery of dexamethasone and moxifloxacin.

To-scale size comparison of a punctum plug depot from Ocular Therapeutix.
Mati Therapeutics is developing the Evolute punctal plug drug delivery system (PPDS), which delivers latanoprost over a period of 2 to 3 months.
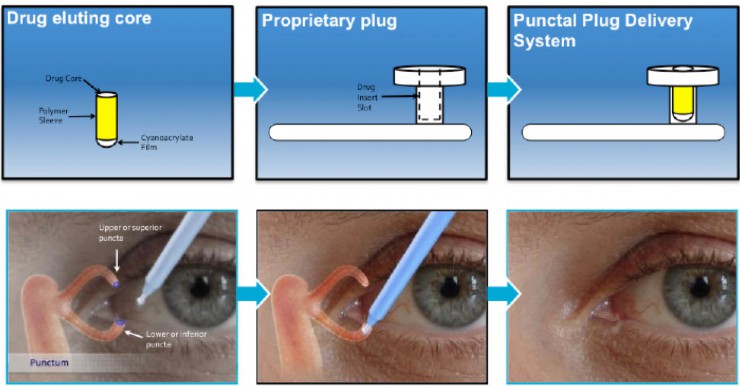
The punctal drug delivery system from Mati Therapeutics.
Amorphex Therapeutics has developed the Topical Ophthalmic Drug Delivery Device, or TODDD, which is a contact lens-like device that sits on the sclera, underneath the upper eyelid, and can hold a large volume of medication or even multiple medications at a time for long-term release. The device is made out of polymers that are biocompatible and well tolerated. The drug can then be delivered over a period of 3 months or more.
Insertion of the Topical Ophthalmic Drug Delivery Device (Amorphex Therapeutics).
OTHER INNOVATIONS
Several other innovations in the delivery of glaucoma medications are emerging.
Pentablock copolymers are biomaterials that can serve as a vehicle for topical and intraocular drug delivery. Nanoparticles of drug can be suspended in the pentablock thermosensitive gel. For glaucoma purposes, a drug, combined with the pentablock copolymer, would be topically applied to the eye and, once exposed to body temperature, would transform from a solution into a gel. The gel stays underneath the eyelid as a film and gradually releases the medication. The copolymer was developed and is being studied by Ashim K. Mitra, PhD, and colleagues at the University of Missouri.
Verisome (Icon Bioscence) is a biodegradable platform being studied for latanoprost delivery over 6 months after injection into the anterior chamber with a 30-gauge needle. The technology is also being investigated for delivery of a medication for retinoblastoma and intravitreal delivery of triamcinolone acetonide.
Pfizer partnered with pSivida (the developer of Iluvien and Retisert) to develop Durasert, which is a bioerodible drug delivery system for latanoprost. Durasert contains latanoprost and can be injected into the subconjunctival space with a 25-gauge needle to provide a low daily dose of the medication for a longer period of time.
DuraSite (InSite Vision) is another vehicle polymer used in commercially available eye drops (Azasite [Inspire Pharmaceuticals] and Besivance [Bausch + Lomb]). DuraSite is currently being studied for bimatoprost topical delivery and was found to improve drug delivery in rabbit eyes compared with Lumigan drops (Allergan).1 It can maintain therapeutic doses of a drug on the ocular surface for up to 6 hours.
PUMP
There is at least one pump system for glaucoma therapy in the pipeline as well. Replenish is developing an ophthalmic micropump system that consists of a small, automated, refillable drug pump that is implanted on the sclera. The pump can inject programmed amounts of drug at set times and can hold up to a year’s worth of medication.
SUMMARY
There are many advantages to delivering glaucoma medical therapy in a reliable and intelligent way: improvement in the patient’s quality of life; better, more continuous IOP control with fewer side effects by utilizing lower doses of medication, etc. Imagine connecting a “smart” drug delivery system to a 24-hour IOP monitor and providing medication in the quantity and frequency needed to halt progression in a particular patient with a specific disease stage and therapeutic needs. I believe it is our generation of ophthalmologists that will get to enjoy a new way to practice glaucoma care!
1. Bowman L, Shafiee A, Hou E, Hosseini K. Ocular pharmacokinetics of ISV-215 (Bimatoprost 0.03%) formulated in a DuraSite delivery system compared to Lumigan in rabbits. Poster presented at: the Association for Research in Vision and Ophthalmology Annual Meeting; May 5-9, 2013; Seattle, Washington.


