
Ocular Therapeutix is a biopharmaceutical company developing first-in-class ophthalmic sealants and sustained drug delivery systems using its proprietary polyethylene glycol (PEG) hydrogel platform technology. Previously approved for use in spinal, neurological, and angioplasty procedures, this technology is being used by Ocular Therapeutix to design therapies specifically for ocular applications. The synthetic nature of the product makes it compatible with a wide range of pharmaceuticals, while the risks of using technologies based on biological components such as collagen or human thrombin materials are eliminated. As a result, these PEG-based materials have undergone extensive biocompatibility and safety testing and enjoy broad market acceptance.
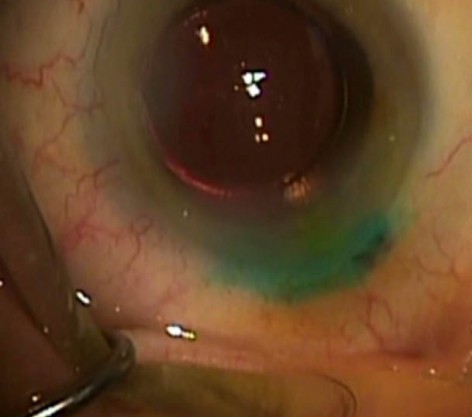
Sealed incision using ReSure Sealant.
HYDROGEL SEALANT
Surgeons may be familiar with Ocular Therapeutix’s ReSure Sealant, the first and only FDA-approved sealant for ophthalmic use. Used to seal corneal incisions after cataract surgery, it is applied to the eye as a liquid and then polymerizes into an adherent hydrogel in less than 30 seconds. The sealant contains a blue colorant that aids in visualization for accurate placement over the incision. The colorant dissipates within hours, leaving a clear sealant on the eye. The product is designed to dissolve or slough off within 7 days, so it does not require removal.
In the pivotal clinical trial, the ReSure Sealant was proven to be superior to sutures for prevention of fluid egress in clear corneal incisions with significantly fewer adverse events.1 The superior seal is most likely attributed to the hydrogel covering the entirety of the incision, rather than point tension placed by the sutures, whereby the incision is still exposed. This method of wound closure is ideal for premium patients, high-risk patients, complicated cataract cases, and patients with Alzheimer disease or dementia.
SUSTAINED-RELEASE DRUG DELIVERY PUNCTUM PLUGS
In addition to its hydrogel sealant, Ocular Therapeutix has several products in the pipeline. The company has been working to encapsulate ophthalmic pharmaceuticals within its hydrogel to deliver sustained and therapeutic levels of drugs to targeted ocular tissues. Inserted noninvasively through the punctum, the plugs reside within the canaliculus during the treatment period and resorb following therapy, so there is no need for removal. The punctum plugs can be tailored to provide the desired duration of therapy—from days to months—and are compatible with ophthalmic pharmaceuticals like prostaglandin analogs, corticosteroids, anti-infectives, and NSAIDs. These sustained-release drug delivery depots may provide more consistent dosing therapy than topical eye drops by avoiding the large variances in drug concentration.
Dexamethasone punctum plug. One of the punctum plugs in Ocular Therapeutix’s pipeline is designed to release dexamethasone to the ocular surface over a 4-week period. The plug was evaluated in a phase 2 clinical trial for the treatment of ocular inflammation and pain following cataract surgery. Results of the study showed that the dexamethasone punctum plug provided statistically significant reductions in inflammation at days 14 and 28 and in pain at all time points. Patients indicated that they could not feel the plug during insertion or postoperatively and were comfortable overall with use of the plug. Given these positive results, this plug is the first of its kind to enter phase 3 trials. Two prospective, multicenter, randomized, double-masked phase 3 trials recently completed enrollment; each will include 240 patients at 17 sites.
Additionally, in mid-November, Ocular Therapeutix announced top-line results from a phase 2 study evaluating its sustained-release dexamethasone plug for the treatment of chronic allergic conjunctivitis. This prospective, multicenter, randomized, double-masked, statistically powered study included 68 patients with the intent to treat for reactions to a variety of allergens over a 42-day period. Patients treated with dexamethasone had statistically significantly lower ocular itching and conjunctival redness scores than the placebo group at all three time points.
Travoprost punctum plug. Ocular Therapeutix is also developing a sustained-release travoprost punctum plug for the treatment of glaucoma and ocular hypertension. This punctum plug is designed to deliver travoprost to the ocular surface for up to 90 days. The company is currently enrolling patients in a phase 2b clinical trial. Previous pilot and phase 2a clinical trials evaluating the travoprost punctum plug showed robust reductions in IOP for up to 3 months, similar to results found with twice-daily timolol dosing.
CONCLUSION
Benefits of sustained ocular drug delivery over topical dosing methods include improved compliance, reduced dosing frequency, continual delivery over time, and reduced patient burden. As patient compliance with ophthalmic pharmaceuticals is always a concern, sustained-release punctum plug therapies may allow for better care of patients in the future.
1. Masket S, Hovanesian JA, Levenson J, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40(12):2057-2066.


