UPDATE ON A GLAUCOMA OUTFLOW PROCEDURE
The Preserflo MicroShunt (Santen, not available in the United States) is a subconjunctival glaucoma implant. The device features a polystyrene tube that is 8.5 mm long; it has an outer diameter of 350 µm and a lumen diameter of 70 µm (Figure).
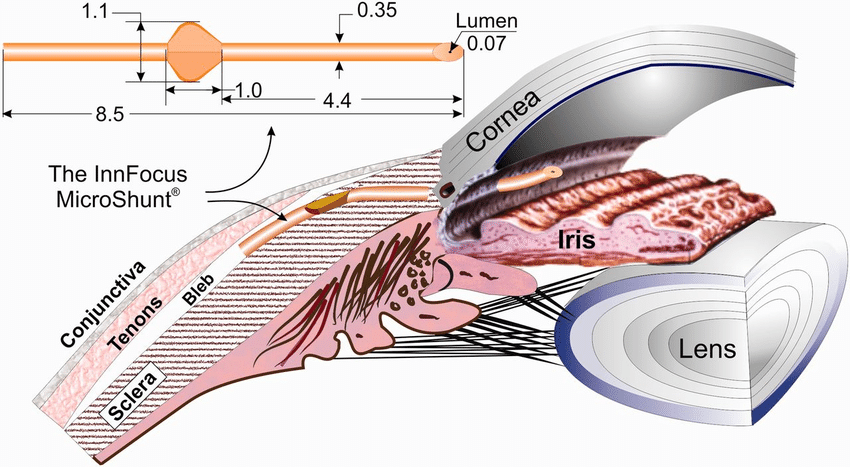
Figure | The Preserflo MicroShunt.
Adapted with permission from Pinchuk L, Riss I, Batlle JF, et al. The use of poly(styrene-block-isobutylene-block-styrene) as a microshunt to treat glaucoma. Regen Biomater. 2016;3(2):137-142.
Douglas Baker, MD, presented 1-year data from a 2-year, randomized multicenter study assessing the safety and efficacy of surgery with the Preserflo compared to trabeculectomy.1 The study enrolled patients with primary open-angle glaucoma and uncontrolled IOP. The primary outcome measure was IOP reduction.
The investigators randomly assigned 395 eyes to receive the device and 132 to undergo trabeculectomy. Surgery was performed with intraoperative mitomycin C 0.2 mg/mL applied with sponges for 2 minutes. The study groups were highly similar; the mean age was 67 years, 58% of patients were phakic, mean diurnal IOP was 21 ±5 mm Hg on an average of 3 ±1 medications, and the visual field mean deviation was 12 ±7 dB.
Overall, 54% of patients in the device group and 73% of patients in the trabeculectomy group achieved the primary endpoint of at least a 20% reduction in mean diurnal IOP without an increase in the number of medications compared to baseline. Among patients with a baseline IOP of 21 mm Hg or higher, the success rate was 64% in the device group and 75% in the trabeculectomy group. One year after surgery, mean IOP was 14.2 mm Hg in the device group and 11.2 mm Hg in the trabeculectomy group on an average of 0.6 and 0.3 medications, respectively. A majority of patients (72% in the device group and 85% in the trabeculectomy group) were free of medication at 1 year.
Complication rates were typical for these types of surgery. Persistent hypotony (IOP ≤ 6 mm Hg at two consecutive visits) occurred in 3% of device eyes and 10% of trabeculectomy eyes; bleb leaks occurred in 9% and 15%, respectively; and choroidal effusions occurred in 5% and 8%, respectively. Endothelial cell loss was low in both groups at 5% and 7%, respectively.
ADHERENCE TO TREATMENT AND GLAUCOMATOUS PROGRESSION
One of many lessons learned from the Collaborative Initial Glaucoma Treatment Study (CIGTS) is that patients’ nonadherence to prescribed medical therapy is associated with glaucomatous vision loss. This aspect of the CIGTS, presented at the AGS meeting and recently published,2 comes from an analysis of 307 patients treated with medication and observed for 7.3 ±2.3 years.
Patients were asked every 6 months, “Did you happen to miss any dose of your medication yesterday?” One hundred forty-two patients (46%) reported never missing a dose of medication, 112 (37%) reported missing medication at up to one-third of visits, 31 (10%) reported missing medication at one-third to two-thirds of visits, and 21 (7%) reported missing medication at more than two-thirds of visits.
Worse medication adherence was associated with worsening visual fields (P = .005). Among patients who reported never missing a dose, the average predicted mean deviation loss over 8 years was 0.62 dB, consistent with age-related loss. Patients who reported missing doses at one-third of visits experienced a loss of 1.42 dB, and those who reported missing doses at two-thirds of visits experienced a loss of 2.23 dB (P < .0001).
Taking a different approach to this question, Donald Fong, MD, and his colleagues at Kaiser Permanente Southern California examined their database of more than 65,000 patients newly diagnosed with open-angle glaucoma over a 10-year period.3 Of these patients, 6,343 met the study criteria, including at least three reliable visual fields.
Pharmacy records were used to determine medication adherence, reported in categories of low, moderate, and high. The average age of patients in the study was 66 ±11 years; 41% were of white, non-Hispanic ethnicity. Baseline disease severity was mild in 17% (mean deviation ≥ -2 dB), moderate in 33% (mean deviation -2 to -6 dB), severe in 16% (mean deviation -6 to -12 dB), and very severe in 9% (mean deviation > -12 dB). Treated patients were observed for an average of 6 ±3 years.
Greater baseline disease severity was associated with better medication adherence. There was a slower rate of glaucomatous progression, however, among patients who demonstrated better adherence over the course of the study (P = .006)
AN INJECTABLE DRUG DELIVERY SYSTEM
The FDA approved a bimatoprost implant (Durysta, Allergan) in March, just after the AGS Annual Meeting. Felipe Medeiros, MD, PhD, presented visual field results from the ARTEMIS phase 3 trials of this intracameral, biodegradable, sustained-release (SR) implant in patients with open-angle glaucoma.4
Investigators randomly assigned patients to treatment with the bimatoprost SR implant or topical timolol 0.5%. Two concentrations of bimatoprost SR were evaluated, 10 µg and 15 µg. Patients in the bimatoprost group received implants every 4 months over the course of 1 year (at baseline, week 16, and week 32). Those in the timolol group administered the drug twice daily. The primary endpoint of the studies was noninferiority to timolol during the 12 weeks after placement of the first implant.
All patients were observed for 52 weeks, with a safety extension to 20 months. From a baseline IOP of 24 mm Hg, during the first 12 weeks, mean diurnal IOP was 16 to 17 mm Hg in eyes that received the implant, which was lower than the mean diurnal IOP in eyes treated with timolol. More than 40% of patients in each group achieved a 30% or greater reduction in IOP at week 12. Endothelial cell loss was 6% at 1 year.
An unexpected finding of the studies was that 80% of the patients observed for 1 year maintained IOP control after the injection of three implants. This effect seemed to be independent of the number of pretreatment medications. Of 68 patients who were using two or more topical drops at screening, 82% maintained IOP control at 1 year with the implant alone.
Dr. Medeiros presented new visual field data from the trials. Testing was performed at baseline, weeks 28 and 52, and study exit. Baseline mean deviation was -2.2 to -2.6 dB. No change was observed after 1 year among patients who received the 10-µg implant. In the timolol group, average mean deviation declined by a statistically significant 0.8 dB. Although the study period was short for detecting glaucomatous progression, this rate of decline is consistent with that previously reported in treated patients.5
LONGER-TERM RESULTS OF MIGS
MIGS combined with cataract surgery has been shown to reduce IOP and medication use. The ultimate goal is preserving vision. A secondary goal for physicians and patients is avoiding additional incisional surgery. Douglas Rhee, MD, presented 4-year outcomes in patients who underwent cataract surgery alone versus in combination with implantation of a Hydrus Microstent (Ivantis).
Five hundred fifty-six patients were randomly assigned to undergo either cataract surgery alone (CS) or in combination with the placement of a Hydrus Microstent (HS). Both groups were similar at screening; the mean number of medications was 1.7 ±0.9, washout diurnal IOP was 25 to 26 mm Hg, and visual field mean deviation was -3.6 dB.
At 4 years, the proportion of eyes requiring no medication was significantly higher in the HS group (65% vs 41%), and mean unmedicated IOP was lower in the HS group (16.7 vs 17.3 mm Hg). There was a significant reduction in the need for incisional glaucoma surgery (1.9% in the HS group vs 6.9% in the CS group). From 2 to 4 years, mean central endothelial cell count decreased by 3% in the HS group and by 2% in the CS group. The investigators attributed the significant reduction in the need for glaucoma surgery (trabeculectomy or tube shunt) despite similar IOP values to the limitations of medical therapy (noncompliance, circadian IOP fluctuation).
1. Baker D, Stiles M, Vold S, et al. Safety and effectiveness of microshunt implantation versus trabeculectomy: one-year results from a randomized, multicenter study. Paper presented at: Annual AGS Meeting; February 27, 2020; Washington, DC.
2. Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2020;127:477-483.
3. Fong D, Shu Y-H, Wu J, et al. Adherence to topical glaucoma medications decreases rate of visual field progression over time. Paper presented at: Annual AGS Meeting; February 29, 2020; Washington, DC.
4. Medeiros F, Benjanian M, Goodkin M, et al. Visual field outcomes in open-angle glaucoma patients treated with bimatoprost SR in phase 3 evaluation: results at primary database lock. Paper presented at: Annual AGS Meeting; February 29, 2020; Washington, DC.
5. Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol. 2013;91:406-412.
6. Rhee D. Reduction in incisional glaucoma surgery after 4 years with a Schlemm’s canal microstent combined with cataract surgery for treatment of primary open angle glaucoma. Poster presented at: Annual AGS Meeting; February 27, 2020; Washington, DC.



