Eye care providers have long recognized Demodex mites as a contributor to ocular surface disease (OSD). With a lack of targeted and effective treatments, however, the problem has not received the full attention it deserves.
Demodex mites, the most common ectoparasite in humans, live on facial skin and eyelids (Figure 1). The two species of these parasites—folliculorum and brevis—are common and usually occur in small numbers.1,2 An infestation of D. folliculorum is marked by several telltale signs of blepharitis, including red, itchy eyelids; irritation; and debris in the eyelashes. D. folliculorum is responsible for 45% of blepharitis cases, with an estimated 9 million patients in the United States affected. However, for the most part, the condition remains underrecognized,3-6 and there is no FDA-approved therapy specifically indicated for Demodex blepharitis.
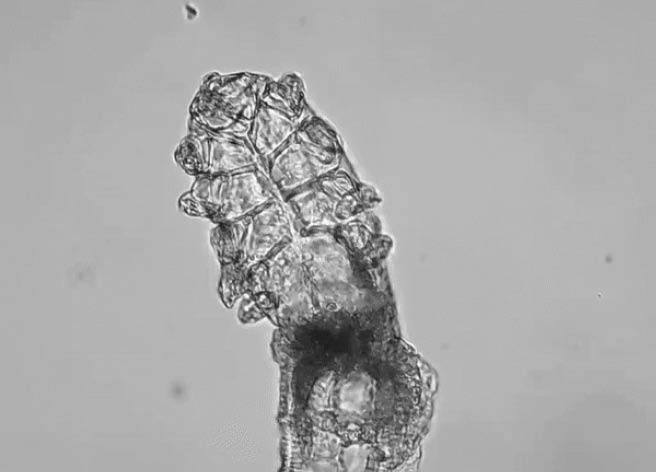
Figure 1 | A Demodex mite is an ectoparasite that lives on human facial skin and eyelids.
INFESTATION INDICATED BY COLLARETTES
An infestation of Demodex can easily be identified by collarettes seen during a routine slit-lamp examination. Typically found at the base of the lash and particularly on the upper lid, collarettes can migrate up the shaft as hair grows.7 The collarettes are formed from partially digested epithelial cells, keratin, and mite waste and eggs that accumulate as mites scratch and feed on the skin. If Demodex are left untreated, patients can experience tear film instability that causes fluctuating and blurred vision, lid and lash abnormalities, and inflammation of the conjunctiva and surrounding skin. As a result, patients may suffer from a host of consequences such as contact lens intolerance and suboptimal surgical outcomes.
Because current therapies used off-label for Demodex blepharitis such as lid hygiene and tea tree oil may be lacking in proven efficacy, eye care providers may be tempted to look past the collarettes. However, due to the multifactorial nature of OSD, if the Demodex component is not eliminated, patients may not experience the improvement in symptoms that they are seeking. Thus, a product that could eradicate an infestation would be a welcome addition to our treatment toolkit for OSD.
THERAPY ATTACKS, KILLS MITES
Tarsus Pharmaceuticals, Inc. is a late-clinical-stage biopharmaceutical company looking to bring a novel therapeutic (TP-03) to market for the safe and effective treatment of Demodex blepharitis. In the company’s Mars and Jupiter studies, which evaluated a total of 75 patients, TP-03 was well tolerated and effective at reducing collarettes and Demodex density with 28 days of treatment and maintained through 90 days.8,9 Statistically significant decreases in collarettes and Demodex density were noted as early as day 14 of treatment. No treatment-related adverse events were observed, and patients reported the drop to be comfortable.
JUPITER STUDY
Design. Two-arm, parallel, single-site study in which 60 patients were randomly assigned 1:1 to active or vehicle control study arms.
Inclusion criteria. Included patients who had at least one eye with more than 10 collarettes present on the upper lid, mild-to-severe lid margin erythema, and an average Demodex density (upper and lower eyelids combined) of 1.5 mites or more per lash (Figure 1).
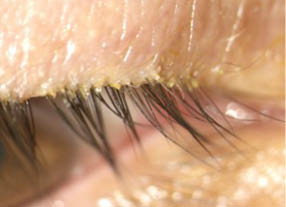
Figure 1 | A baseline image from day 1 of the Jupiter study shows an eyelash/eyelid margin with visible collarettes.
Dosing. Participants administered one drop of their assigned treatment in each eye twice daily for 28 days, and they were assessed at days 7, 14, 28, 60, and 90.
Efficacy and safety. Efficacy was determined by the decrease in the signs of Demodex blepharitis (collarettes) and the decrease in the Demodex density (mites per lash). Safety was determined by assessing treatment-related adverse events and by evaluating any changes in visual acuity, IOP, and slit-lamp biomicroscopy findings, including corneal staining.
Results. This study showed that use of TP-03 (Tarsus Pharmaceuticals) was well tolerated and effective in reducing collarettes and Demodex density through 90 days in patients with Demodex blepharitis. The change in collarette grade demonstrated statistically significant decreases for both eyes (upper and lower eyelid margins) compared with control and persisted for 2 months after treatment. There was also a statistically significant decrease in mean mite density compared with control in both eyes beginning at day 28 and persisting an additional 2 months after treatment (Figure 2). No treatment-related adverse events occurred, and patients reported the drop to be comfortable.
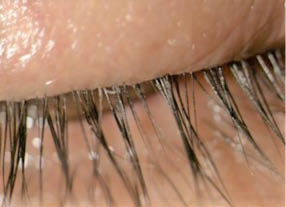
Figure 2 | An image from the Jupiter study shows the eyelash/eyelid margin at day 28.
If approved, TP-03 will be one of the few therapies for OSD that allows eye care providers to attack an underlying cause of disease in a definitive and hopefully curative way. Demodex blepharitis can be seen in isolation, but often it is a component of the larger OSD picture. We may not be putting the importance of these mites in the proper context.
CONCLUSION
TP-03 would likely be used in conjunction with other medications and device-based treatments according to a patient’s underlying disease process. However, the elimination of a Demodex infestation alone should improve symptomatology and increase comfort for patients with dry eye and blepharitis.
1. English FP. Demodex folliculorum and oedema of the eyelash. Br J Ophthalmol. 1971;55(11):742-746.
2. Rufli T, Mumcuoglu Y. The hair follicle mites Demodex folliculorum and Demodex brevis: biology and medical importance. A review. Dermatologica. 1981;162(1):1-11.
3. AAO Preferred Practice Patterns Corneal/External Disease Committee: Amescua G, Akpek EK, Farid M, et al. Blepharitis PPP-2018. AAO. November, 2018. www.aao.org/preferred-practice-pattern/blepharitis-ppp-2018. Accessed October 27, 2020.
4. Coston TO. Demodex folliculorum blepharitis. Trans Am Ophthalmol Soc. 1967;65:361-392.
5. Gao Y-Y, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Opthalmol Vis Sci. 2005;46(9):3089.
6. Zhao YE, Wu LP, Hu L, Xu JR. Association of blepharitis with Demodex: a meta-analysis. Ophthalmic Epidemiol. 2012;19(2):95-102.
7. Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom. 2018;10:57-63.
8. Gonzalez-Salinas R, Ramos-Betancourt N, Corredor-Ortega C, et al. Pilot study to evaluate the safety and efficacy of TP-03 for the treatment of blepharitis due to Demodex infestation (Mars study). Paper presented at: the ARVO Annual Meeting; 2020.
9. Hom MH, Ceballos JC, Massaro-Corredor M, et al. Randomized controlled trial to evaluate the safety and efficacy of TP-03 for the treatment of blepharitis due to Demodex infestation (Jupiter study). Poster presented at: Optometry’s Meeting; 2020.



