Glaucoma can be a challenging disease to treat, and we do well to utilize all of the tools at our disposal. When treating glaucoma medically, we use many different medications and, broadly speaking, employ agents that both enhance the outflow of aqueous and suppress its production.
It is puzzling to me that glaucoma surgeons generally favor surgical interventions that enhance aqueous outflow. The rationale is that glaucoma is a disease of impaired aqueous outflow and that we should bypass the pathology. Although this argument is somewhat compelling, I am reminded that, at least anatomically, most glaucoma patients have normal-appearing outflow pathways. Given the popularity of aqueous suppressants in topical therapy, why do we apply this dogma to surgical treatment but not to our medical management?
People still go blind from glaucoma every day, and I believe we could use all of the help treating this challenging disease that we can get. A series of prospective surgical clinical trials have painted the landscape. In the tube versus trabeculectomy study,1-2 212 previously operated eyes of 212 patients were randomized to either receive a 350 mm2 Baerveldt implant (Abbott Medical Optics) or undergo trabeculectomy with 0.4 mg/mL mitomycin C for 4 minutes. The investigators demonstrated that after cataract surgery or a failed trabeculectomy, tube shunt surgeries had a more durable pressure lowering, with 70% survival at 5 years compared with trabeculectomy.1-2
The Ahmed Baerveldt comparison study3 demonstrated that the Ahmed valve (New World Medical) is a safer drainage device than the Baerveldt, with a serious complication rate (requiring reoperation or associated with more than two lines of vision loss) of 20% in the Ahmed group compared with 34% in the Baerveldt group. However, the authors also found that that the Baerveldt implant lowered pressure slightly better by 5 years. I have migrated many of my surgeries over to the Ahmed valve but, at times, have desired greater IOP lowering. Enter ECP.
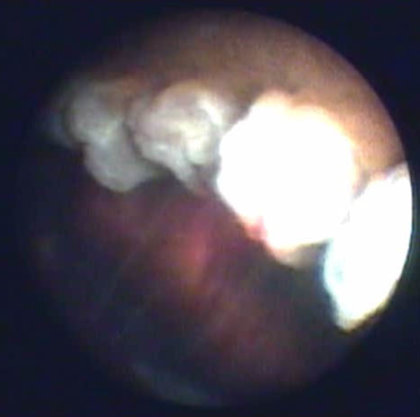
Endoscopic view of a ciliary process receiving treatment from the 810-nm laser.
ENDOSCOPIC CYCLOPHOTOCOAGULATION
There are several potential advantages of decreasing the production of aqueous humor. Cyclophotocoagulation refers to the thermal targeting of ciliary body pigment epithelial cells to reduce the amount of aqueous humor produced. Endoscopic cyclophotocoagulation (ECP) is an ab interno (rather than transscleral) approach for cycloablation that utilizes endoscopy with an 810-nm diode laser (Beaver-Visitec Endo Optiks). It is one of the few glaucoma procedures that can be repeated and titrated without leaving any conjunctival scars; it also does not alter the outflow pathway anatomy in any way that would eliminate future glaucoma surgical options.
Gayton and colleagues prospectively randomized 58 eyes of 58 patients to phacotrabeculectomy versus phaco/ECP and found that 30% of ECP-treated patients achieved IOP below 19 mm Hg without medication and 65% achieved an IOP below 19 mm Hg with medication, as opposed to 40% without medication and 52% with medication in the trabeculectomy group.4 Kahook and colleagues followed 25 patients with a baseline IOP of 24.48 mm Hg after ECP; after 6 months, the IOP was 13.00 mm Hg (a 47% reduction), with reduced medication usage from 2.56 to 0.52 bottles per patient.5 One thing I noticed from performing cyclophotocoagulation on eyes that had undergone previous drainage device surgery was that cyclophotocoagulation seems to work better if a glaucoma drain is in place, even if that drain has apparently failed.
Why not perform ECP at the time that the drain is placed? Hypotony could occur if a nonvalved drain is used, but, at least theoretically, the Ahmed valve should protect against this.
THE ACE PROCEDURE
Several years ago, I began performing ECP at the same time as Ahmed valve placement in pseudophakic eyes with severe glaucoma. If the eye had a visually significant cataract, I would remove the cataract, perform ECP, and place the Ahmed valve in a procedure that I affectionately call the ACE procedure (Ahmed, Cataract, ECP).
The technique.
I begin by performing a standard cataract extraction, and I inflate the ciliary sulcus with viscoelastic after placement of the IOL. I perform the dissection and place the Ahmed valve plate, entering the eye with a 23-gauge needle through a tunneled approach about 4 mm back from the limbus, to deliver the tube into the posterior chamber or iridociliary sulcus. I trim the tube and place it into the posterior chamber. Now, when I perform ECP, I can visualize the tube placement in the sulcus to confirm that it is well positioned.
The trickiest part of the procedure is reforming the anterior chamber and removing the appropriate amount of viscoelastic from the sulcus. I leave the eye with a good amount of viscoelastic in it to prevent hypotony and enough viscoelastic in the anterior chamber to keep it formed. Ironically, I end up giving the patient oral Diamox (acetazolamide) for the first night or day after surgery to essentially slow aqueous production and allow the viscoelastic to stay in the eye for the first few postoperative days. I finish the case with a subconjunctival steroid injection to decrease inflammation.
RESULTS
Although I am still waiting to formally review my results, I can offer some impressions from my first 100 surgeries. I have not had a single case of hypotony with the ACE technique. Just as with the Ahmed valve by itself, I have seen some failures and have had a few patients go on to receive additional tube shunts or transscleral laser. However, most patients do very well, achieving pressures in the low teens and the ability to discontinue many pressure-lowering agents.
Case report.
Recently, a woman presented with visual acuity of bare light perception in her right eye (OD) and light perception in her left eye (OS). She was taking four topical glaucoma medications, and her IOP was 42 mm Hg OD and 32 mm Hg OS. She had brunescent cataracts, and there was no view to the back of her eye, but the ultrasound demonstrated normal anatomy.
I performed an ACE procedure on both eyes. At her 6-month follow-up, her visual acuity was hand motions OD and 20/400 OS. She is still taking three topical agents, but her IOP has been reduced to 13 mm Hg OD and 12 mm Hg OS. The procedure has had a major impact on her vision, her IOP, and her quality of life.
SUMMARY
I believe we are entering the era of combined-mechanism glaucoma surgery. Enhancing the efficacy of outflow procedures with ECP is a great approach, and I am hopeful that the dogma of outflow-only surgery will yield to this promising new technique.
1. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; for the Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789-803.
2. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC; for the Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.
3. Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; for the Ahmed Baerveldt Comparison Study Group. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118(3):443-452.
4. Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma. 2007;16:527-530.
5. Gayton JL, Van Der Karr M, Sanders V. Combined cataract and glaucoma surgery: trabeculectomy versus endoscopic laser cycloablation. J Cataract Refract Surg. 1999;25:1214-1219.


