Keratoconus affects more than 160,000 people in the United States. Its prevalence is estimated to be higher in other parts of the world, including Asia and the Middle East, and its incidence seems to be increasing globally.
The current FDA-approved treatment option for keratoconus is corneal crosslinking involving removal of the epithelium, followed by application of riboflavin and UV light. Although efficacious, this procedure carries certain disadvantages, including pain, corneal haze, and risk of infection and scarring. Additionally, keratoconus is a chronic disease, and regression is possible with a one-time surgical treatment.
Innovation on the Horizon: Eye Drop That Induces Crosslinking
IVMED-80
In pursuit of a treatment option independent of surgery and laser treatment, iVeena Delivery Systems developed a product called IVMED-80, which is a twice-daily eye drop for the treatment of keratoconus. IVMED-80 was granted orphan drug designation by the FDA in 2017.
HOW IT WORKS
Keratoconus is a complex multifactorial disease; however, several strong links across multiple studies indicate that mutations in the lysyl oxidase (LOX) gene may be involved in its pathogenesis.1-7 LOX is an innate enzyme in the cornea that mediates physiologic crosslinking as a person ages. Interestingly, LOX is a copper-dependent amine oxidase, and copper has been found to be lower in keratoconic corneas than in normal corneas.8
Thus, we hypothesized that a copper deficiency in the central cornea likely inhibits LOX activity and leads to insufficient native corneal collagen crosslinks. IVMED-80, which is a copper derivative, was designed to induce up-regulation of LOX and thereby strengthen corneal fibers.
In addition to keratoconus, IVMED-80 has applications in preventing post-LASIK ectasia and treating pellucid marginal degeneration and other diseases associated with corneal thinning.
CLINICAL DATA
To investigate IVMED-80, we cultured fibroblasts from normal human corneas and keratoconic corneas. When we looked at the LOX activity, we observed that normal corneas had a much higher expression at baseline than keratoconic corneas. Further, treatment with IVMED-80 dramatically increased LOX activity in cultures from normal and keratoconic fibroblasts, providing support for the eye drop’s mechanism of action.
In a study of rabbit corneal biomechanics with twice daily administration of IVMED-80, we observed that the corneal elastic modulus in the rabbits increased within 1 week of treatment to a level comparable to that at 1 year after conventional crosslinking (Figure 1). This was then correlated in human cadaver corneas; we analyzed two pairs of donor corneas and found that the untreated donor corneas had an inferior stress-strain curve to their mates treated with IVMED-80. The drop was well tolerated in rabbits, and there were no abnormal inflammation or findings in the cornea, the retina, or systemic organs. The rabbits did not show any inflammation or evidence of pain.
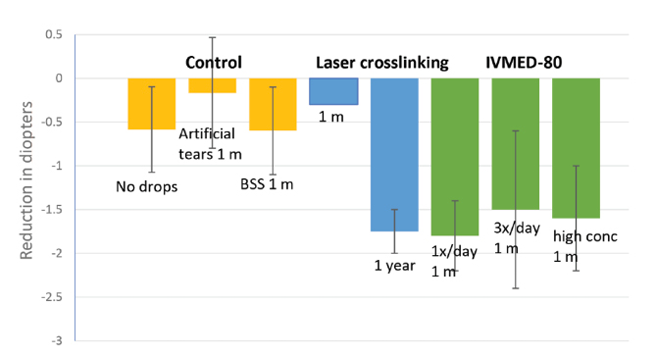
Figure 1 | IVMED-80 was effective in decreasing corneal diopter measurements in rabbits compared with control treatments. The eye drop achieved improvement in corneal elastic modulus within 1 to 4 weeks of therapy to a level comparable to conventional corneal crosslinking at 1 year after laser surgery in rabbits.
Using a custom topography unit for assessing rabbit corneas, we also found that three different dosing regimens—once daily, three times daily, or at high concentration three times daily—achieved 1.50 to 1.70 D of corneal flattening after 1 month of treatment with IVMED-80. This is comparable to what is observed in rabbit and human eyes at 1 year after conventional crosslinking and was significantly higher than in any of the control groups.
To note, some of these experiments were supported by a National Institutes of Health Small Business Innovation Research grant, with principal investigator Sarah Molokhia, PhD (Topical Eyedrop Therapy for Keratoconus; R43EY027636).
ON THE HORIZON
Longer group studies of IVMED-80 are under way. We have observed that the amount of corneal flattening increases to about 2.00 D at 3 months of treatment and that, if we withdraw the treatment after 2 or 3 months, some rebound occurs. Therefore, we are working to determine the optimal duration of the treatment and how much total corneal flattening can be achieved with the IVMED-80 eye drop.
1. Bykhovskaya Y, Li X, Epifantseva I, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012;53(7):4152-4157.
2. Dudakova L, Liskova P, Trojek T, Palos M, Kalasova S, Jirsova K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res. 2012;104:74-81.
3. Zhang J, Zhang L, Hong J, Wu D, Xu J. Association of common variants in LOX with keratoconus: a meta-analysis. PLoS One. 2015;10(12):e0145815.
4. Shetty R, Rajiv Kumar N, Pahuja N, et al. Outcomes of corneal cross-linking correlate with cone-specific lysyl oxidase expression in patients with keratoconus. Cornea. 2018;37(3):369-374.
5. Shetty R, Sathyanarayanamoorthy A, Ramachandra RA, et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol Vis. 2015;21:12-25.
6. Wojcik KA, Blasiak J, Szaflik J, Szaflik JP. Role of biochemical factors in the pathogenesis of keratoconus. Acta Biochim Pol. 2014;61(1):55-62.
7. Hasanian-Langroudi F, Saravani R, Validad MH, Bahari G, Yari D. Association of lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet. 2015;36(4):309-314.
8. Dudakova L, Liskova P, Jirsova K. Is copper imbalance an environmental factor influencing keratoconus development? Med Hypotheses. 2015;84(5):518-524.