Even with the many advances made in refractive and corneal surgery in the past decade, ophthalmic surgeons commonly face challenges. These can include dealing with patients with suspicious topographic patterns or trying to combine subjective interpretation of topographic maps with the innumerable indices that have been described to estimate the risk of ectasia.1
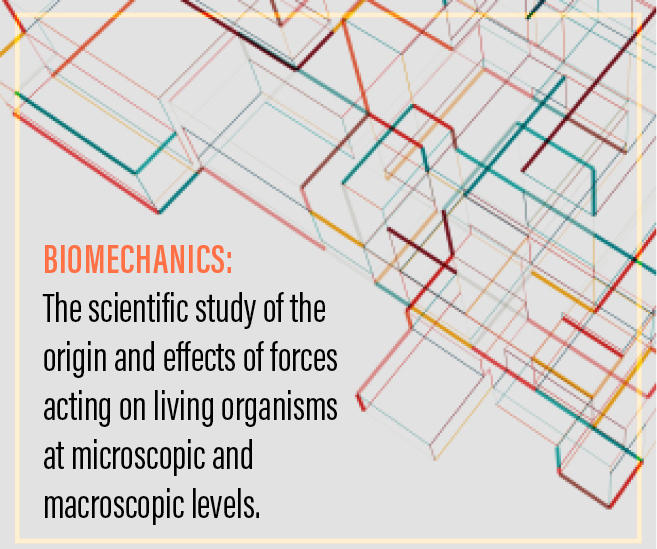
The point of convergence of these variables is the biomechanical structure of the cornea.2,3 Biomechanics is the scientific study of the origin and effects of forces acting on living organisms at microscopic and macroscopic levels. The topographic, pachymetric, and elevation alterations that clinicians see in the eyes of their patients with keratectasia are secondary to focal changes in structure. These structural changes lead to modifications in the interactions of the elements that compose the stroma, thereby altering the biomechanical characteristics of the cornea.4
The complexity of the cornea and of its interaction with adjacent structures prevents a single number or index from representing its elastic behavior.5,6 In assessing the biomechanics of the cornea, the ocular structures neighboring the cornea must also be taken into account. It is not possible to make a complete evaluation of corneal elasticity without considering IOP values and the characteristics of the limbal region, where the cornea’s collagen fibers are anchored. Other factors, such as regional differences within the corneal stroma itself, must also be factored in.7
The gold-standard method for measuring the biomechanical characteristics of the cornea is extensiometry, the measurement of extension under an applied stress. In this laboratory evaluation, both ends of a strip of tissue are attached to a device and tensioned, or stretched, to establish the relationship between stress and strain.8 This method has identified significant deficits in elastic tension in keratoconic corneas as well as variations in elastic moduli throughout the stroma (anterior > posterior, central > periphery). These findings may have a diagnostic role in clinical practice.9
Aiming to apply these concepts in the clinic, devices such as the Ocular Response Analyzer (Reichert Technologies) and the Corvis ST (Oculus Optikgeräte) were developed to diagnose structural changes in the cornea earlier than could be detected with conventional methods.10 Both technologies use a jet of air to deform the cornea and provide indices to help diagnose ectatic diseases. It is worth mentioning that these variables are provided as a measure of the whole cornea, with no direct relation to the elastic modulus.11 Studies have shown that both devices can differentiate normal corneas from those with ectatic disease. However, in borderline keratoconus suspect cases, both have low sensitivity and specificity.12,13
BRILLOUIN MICROSCOPY AND OPTICAL COHERENCE ELASTOGRAPHY
In order to assess Young’s modulus in vivo, two technologies are being investigated: Brillouin microscopy and optical coherence elastography.14,15 Brillouin microscopy utilizes a spectrometer capable of detecting a specific light-scatter particle, which is directly related to the elastic modulus of the sample being analyzed. Ex vivo and in vivo studies have shown promising results, and prototypes are being tested in clinical sites in the United States and Europe.16,17
Optical coherence elastography uses a different technology, based on a dynamic applanation device attached to a spectral-domain OCT unit. In the bioengineering laboratory of the Cleveland Clinic, researchers developed a prototype that uses the technique of elastography by OCT to measure small displacements within the corneal stroma after a small applanation, similar to Goldmann tonometry. Investigators have reported on the use of this approach in several experimental scenarios,18 and the technology is currently being evaluated in several in vivo studies, the results of which will soon be published.
BIOMECHANICAL MODELS
Also being developed and tested at multiple clinical sites are biomechanical models that use mathematical expressions to describe the response of a material to a disturbance.19
One of the methods that can be used to study complex structures such as the cornea is the finite element method. The predictive value of any model depends on the premises and the quality of the values input for each element. Therefore, to develop highly accurate models, advanced and reproducible anterior segment data must be acquired.
The objective is to be able to anticipate the results of refractive surgery in advance by performing individualized analysis and simulations of refractive surgery (in silico surgery) for each patient.20 This would allow ophthalmologists to adjust their nomograms based not only on the characteristics of the excimer laser and the technique used for correction but also on the specific tomographic and biomechanical characteristics of the patient.21
CONCLUSION
It is important to emphasize that no single index described to date can accurately characterize corneal biomechanics in vivo. The future of refractive surgery will be the ability to simulate the outcome of each patient’s surgery beforehand, allowing necessary adjustments to obtain the best possible result in an individualized way. This will be possible only with a complete understanding of the relationship between corneal structure and function. Methods such as OCT elastography and Brillouin microscopy will lead to a deeper understanding of the cornea’s biomechanical structure in vivo, providing new evidence for the early diagnosis of ectatic diseases and assisting surgeons in choosing the best approach for each patient.
1. Giri P, Azar DT. Risk profiles of ectasia after keratorefractive surgery. Curr Opin Ophthalmol. 2017;28(4):337-342.
2. Roberts CJ. Concepts and misconceptions in corneal biomechanics. J Cataract Refract Surg. 2014;40(6):862-869.
3. Girard MJA, Dupps WJ, Baskaran M, et al. Translating ocular biomechanics into clinical practice: current state and future prospects. Curr Eye Res. 2015;40(1):1-18.
4. Dupps WJ. Biomechanical modeling of corneal ectasia. J Refract Surg. 2005;21(2):186-190.
5. Boote C, Elsheikh A, Kassem W, et al. The influence of lamellar orientation on corneal material behavior: biomechanical and structural changes in an avian corneal disorder. Invest Opthalmol Vis Sci. 2011;52(3):1243-1251.
6. Winkler M, Chai D, Kriling S, et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci. 2011;52(12):8818-8827.
7. Morishige N, Wahlert AJ, Kenney MC, et al. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest Ophthalmol Vis Sci. 2007;48(3):1087-1094.
8. Hjortdal JO. Extensibility of the normo-hydrated human cornea. Acta Ophthalmol Scand. 1995;73(1):12-17.
9. Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF. Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008;24(1):S85-S89.
10. Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31(1):156-162.
11. De Stefano VS, Dupps WJ. Biomechanical diagnostics of the cornea. Int Ophthalmol Clin. 2017;57(3):75-86.
12. Fontes BM, Ambrósio R, Jardim D, Velarde GC, Nosé W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology. 2010;117(4):673-679.
13. Fontes BM, Ambrósio Jr R, Velarde GC, Nosé W. Corneal biomechanical evaluation in healthy thin corneas compared with matched keratoconus cases. Arq Bras Oftalmol. 2011;74(1):13-16.
14. Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Invest Ophthalmol Vis Sci. 2012;53(1):185-190.
15. Ford MR, Dupps WJ, Rollins AM, Roy AS, Hu Z. Method for optical coherence elastography of the cornea. J Biomed Opt. 2011;16(1):0160051-0160057.
16. Scarcelli G, Besner S, Pineda R, Yun SH. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest Opthalmol Vis Sci. 2014;55(7):4490-4495.
17. Scarcelli G, Yun SH. In vivo Brillouin optical microscopy of the human eye. Opt Express. 2012;20(8):9197-9202.
18. Ford MR, Roy AS, Rollins AM, Dupps WJ. Serial biomechanical comparison of edematous, normal, and collagen crosslinked human donor corneas using optical coherence elastography. J Cataract Refract Surg. 2014;40(6):1041-1047.
19. Sinha Roy A, Dupps WJ. Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci. 2011;52(12):9174-9187.
20. Seven I, Vahdati A, De Stefano VS, Krueger RR, Dupps WJ. Comparison of patient-specific computational modeling predictions and clinical outcomes of LASIK for myopia. Invest Opthalmol Vis Sci. 2016;57(14):6287-6297.
21. Dupps WJ, Seven I. A large-scale computational analysis of corneal structural response and ectasia risk in myopic laser refractive surgery. Trans Am Ophthalmol Soc. 2016;114(T1):1-16.




